All of the major points of this information sheet contain a number of stated or implied inaccuracies. Hence, we will consider each of its principal "bullets" in detail.
· Some agricultural regions will be threatened by climate change, while others may benefit.
This statement is certainly possible, but another is much more probable, based on the contents of the peer-reviewed scientific literature: Few agricultural regions will be threatened by climate change, while most should benefit. Whether or not climate change occurs, the CO2 content of the air will rise appreciably. This phenomenon will enhance the productivity of nearly all crops (Kimball, 1983a, b; Cure and Acock, 1986; Mortensen, 1987; Lawlor and Mitchell, 1991); and it will have the greatest percentage effect on vegetation exposed to less-than-favorable growing conditions, including stresses imposed by high temperature, soil salinity, aerial pollutants, lack of sunlight and insufficient water (Idso and Idso, 1994).
· Climate and agricultural zones are likely to shift towards the poles.
This statement is based on the prediction that air temperature increases
will be greater near the poles than at the equator; and it assumes
that such a change in climate would require a poleward shift in intensive
agriculture if optimal production is to be maintained. This assumption,
however, ignores the fact that the optimum temperature for plant growth
and development typically rises with increasing levels of atmospheric
CO2 (Berry and Bjorkman, 1980; McMurtrie and Wang,
1993; Cowling et al., 1998), a phenomenon that has been clearly elucidated
by Long (1991), who calculated from well-established plant physiological
principles that the optimum growth temperatures of most C3
plants should rise by approximately 5°C for a 300 ppm increase in the
air's CO2 content. And Long's conclusions have
been substantiated by a number of studies (Bjorkman et al., 1978;
Nilsen et al., 1983; Jurik et al., 1984; Seeman et al.,
1984; Harley et al., 1986; Stuhlfauth and Fock, 1990; McMurtrie et
al., 1992) that have experimentally demonstrated that a 300 ppm
increase in atmospheric CO2 causes the optimum growth
temperatures of C3 plants to rise by an average of
nearly 6°C (Idso and Idso, 1994).
Clearly, this effect would more than compensate for the CO2-induced air temperature rise predicted to occur by most climate models. In fact, the photosynthetic rates of the C3 plants described above were found to be nearly two times greater at the warmer optimum temperatures characteristic of a 300 ppm CO2-enriched atmosphere than the rates that prevailed at the cooler optimum temperatures characteristic of ambient atmospheric CO2 concentrations (Idso and Idso, 1994). Consequently, not only would typically predicted increases in atmospheric CO2 and air temperature not hurt agricultural plants, they would actually promote their growth and development. And this beneficial phenomenon would occur right where the plants are growing today, providing no impetus for any poleward shift in agricultural production zones, although high-latitude warming would provide that option. More likely, however, it would serve as a basis for expanding (as opposed to shifting) agricultural production, which may well be needed in a world of ever-increasing human population.
· Soil moisture will be affected by changing precipitation patterns.
This statement may well be true, but it is misleading in that it raises
the specter of more frequent and severe droughts that could significantly
reduce the productivity of important agricultural areas. Although
it is conceivable that some regions may become drier in a high-CO2
world, the climate models themselves predict an intensification of
the planet's hydrologic cycle as the air's CO2 content
rises. Furthermore, most plants exhibit decreased stomatal
conductances at elevated CO2 (Kimball and Idso,
1983; Morison, 1987; Field et al., 1995; Rey and Jarvis, 1998), which
reduces plant evaporative water losses via transpiration
(Overdieck and Forstreuter, 1994; Sgherri et al., 1998; Tognetti
et al., 1998). Together with the increases in plant productivity
that are driven by elevated CO2, these water-saving
effects of atmospheric CO2 enrichment greatly enhance
plant water-use
efficiency (Rogers et al., 1983; Idso et al., 1985; Valle
et al., 1985; Fernandez et al., 1998); and the benefits provided
by this proven consequence of atmospheric CO2
enrichment are more than sufficient to compensate for the worst-case scenarios
of possible (but unlikely) regional rainfall reduction predicted by the
climate models (Idso, 1989).
· Higher temperatures will influence production patterns.
The tone of this section of the information sheet is largely pessimistic. However, if the rising concentration of atmospheric CO2 is accompanied by an increase in air temperature, the productivity of most plants should rise substantially. In a comprehensive analysis of 42 different experiments, for example, Idso and Idso (1994) found that the percentage growth enhancement resulting from a 300 ppm increase in the air's CO2 content became progressively larger with increasing air temperature, going from approximately 20% at 15°C to 100% at 38°C.
This increase in relative growth response arises from the fact that the growth-retarding process of photorespiration, which is most pronounced at high temperatures (Hanson and Peterson, 1986), is effectively inhibited by atmospheric CO2 enrichment (Grodzinski et al., 1987). So powerful is this effect of elevated CO2, in fact, that the optimum temperature for plant growth and development typically rises with increasing levels of atmospheric CO2 (Berry and Bjorkman, 1980; McMurtrie and Wang, 1993; Cowling et al., 1998). Consequently, if atmospheric CO2 and air temperature rise together in the future, they will likely work synergistically to promote even better plant growth and development, as new investigations continue to demonstrate (Vu et al., 1997; Hakala, 1998; Bunce et al., 1998; Reddy et al., 1998).
· More carbon dioxide in the atmosphere could boost productivity.
Not only could it; it most likely will. Kimball (1983a, b), for example, conducted two of the earliest analyses of the peer-reviewed scientific literature dealing with plant responses to atmospheric CO2 enrichment. From 770 individual plant responses, he determined that a 300 ppm rise in the air's CO2 content boosted the productivity of most herbaceous plants by approximately 33%. Other reviews conducted by Cure and Acock (1986), Mortensen (1987) and Lawlor and Mitchell (1991) have produced similar results.
Perhaps the largest such review ever conducted was that of Idso (1992), which utilized papers published over the decade subsequent to the reviews of Kimball. This comprehensive assessment of the pertinent literature incorporated a total of 1,087 observations of plant responses to atmospheric CO2 enrichment obtained from 342 peer-reviewed scientific journal articles authored by 484 scientists residing in 27 foreign countries and 27 American states, representing 24 universities, 30 American government research organizations and 88 foreign institutions. 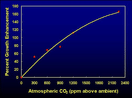 From this vast array of studies, it was determined that 93% of the plant responses to atmospheric CO2 enrichment were positive, 5% were negligible, and only 2% were negative. The mean growth response curve of the plants investigated in these studies is depicted in the accompanying figure, which shows the percentage increase in plant growth as a function of increases in the air's CO2 content. In viewing this comprehensive result, one simple fact stands out clear and unmistakable: nearly all plants grow better with more CO2 in the air.
From this vast array of studies, it was determined that 93% of the plant responses to atmospheric CO2 enrichment were positive, 5% were negligible, and only 2% were negative. The mean growth response curve of the plants investigated in these studies is depicted in the accompanying figure, which shows the percentage increase in plant growth as a function of increases in the air's CO2 content. In viewing this comprehensive result, one simple fact stands out clear and unmistakable: nearly all plants grow better with more CO2 in the air.
Productivity increases, however, can vary by plant type. Poorter (1993), for example, found the average growth stimulation of a 300 ppm increase in atmospheric CO2 to be 41% for 130 different C3 plants, 22% for nine C4 species, and 15% for six CAM plants; and in this regard we note that over 95% of earth's plants are C3 species, 3 to 4% are CAM, and less than 1% are C4. In terms of sub-groups, C3 trees appear to be more responsive to atmospheric CO2 enrichment than any other types of plants. Poorter (1993), Ceulemans and Mousseau (1994) and Wullschleger et al. (1995, 1997) reported the results of 176 experiments on trees and other woody plants that reveal a mean growth enhancement of 48% for a 300 ppm increase in the air's CO2 content. And preceding them, Idso (1992) reviewed 181 tree experiments that exhibited a mean growth enhancement of 62% for a 300 ppm increase in the air's CO2 content.
· The productivity of rangelands and pastures would also be affected.
Again, the tone of this section is somewhat negative, clearly without cause;
for the biological benefits of atmospheric CO2 enrichment
described in the preceding sections will apply to these ecosystems as well
as any other. In some instances, in fact, they could be extremely
beneficial, as in the case of nutrient-poor chalk grasslands in Europe.
In experimental plots there, the CO2-induced growth
stimulation of three perennial
plants (two forbs and a grass) was only moderate, as long as the well-being
of the plants was not challenged. Following a simulated grazing event,
however, all three species displayed 30 to 40% increases in net photosynthesis
when grown at elevated CO2, suggesting that atmospheric
CO2 enrichment helps plants better withstand, or even
recover from, the debilitating effects of having their foliage eaten by
livestock or pests, even under less-than-optimum conditions of soil fertility
(Bryant et al., 1998).
· The global yield from marine fisheries should remain unchanged by global warming.
Although this statement is not optimistic, neither is it negative, as are nearly all of the other points of this and all the other information sheets. However, it is important to note that in an analysis of data from 22 aquatic ecosystems, of both fresh and salt water composition, Cyr and Pace (1993) demonstrated that herbivore biomass rises linearly in response to increases in net primary production, which is nearly always stimulated by atmospheric CO2 enrichment. Hence, as the air's CO2 content rises in the future, it is likely that the world's waters will be able to support even greater fish populations then they do today.
· Food security risks are primarily local and national. Although this information section acknowledges that global agricultural production should be able to maintain itself over the next century, it suggests that food production could falter at the regional level due to various environmental stresses. However, the effects of less-than-favorable growing conditions imposed by resource limitations and environmental stresses have been clearly demonstrated to not destroy the stimulation of plant productivity provided by the aerial fertilization effect of atmospheric CO2 enrichment (Idso and Idso, 1994). In fact, the CO2-induced percentage increase in plant productivity is often greater under unfavorable growing conditions. Consequently, agricultural production will likely increase nearly everywhere as the CO2 content of the air continues to rise.
· The most vulnerable people are the landless, poor, and isolated.
This section suggests that people "dependent on isolated agricultural systems to produce food in semi-arid and arid regions" will be most at-risk in terms of their ability to produce food. However, as noted in the preceding section, just the opposite is more likely to occur: poor and isolated people in arid and semi-arid lands will probably benefit, and benefit more than anyone else.
With more CO2 in the air, for example, crops typically produce more biomass. Moreover, on a per-unit-leaf-area basis, they lose less water via transpiration, as they tend to have lower stomatal conductances at elevated CO2. Hence, the amount of carbon gained per unit of water lost per unit leaf area -- or water-use efficiency -- should increase dramatically as the air's CO2 content rises (Fernandez et al., 1998; Rey and Jarvis, 1998; Tognetti et al., 1998). And nowhere would this phenomenon have a greater positive impact than in the subsistence agricultural communities of the earth's arid and semi-arid regions.
· Effective policies can help to improve food security.
Yes, effective policies can help a lot; but they can only do so much, and they don't come easy, and they don't come free. Fortunately, the aerial fertilization effect of the steadily rising atmospheric CO2 concentration will be much more accessible and pervasive. Undaunted by national and international borders, without regard for policies or politics, blind to race, gender and every human prejudice, the ongoing rise in the air's CO2 content will automatically, and in the face of every effort to prevent it, slowly but surely and ever-increasingly boost the productivity of nearly every crop imaginable across the entire globe.
References
Arp, W.J., Van Mierlo, J.E.M., Berendse, F. and Snijders, W. 1998. Interactions between elevated CO2 concentration, nitrogen and water: effects on growth and water use of six perennial plant species. Plant, Cell and Environment 21: 1-11.
Berry, J. and Bjorkman, O. 1980. Photosynthetic response and adaptation to temperature in higher plants. Annual Review of Plant Physiology 31: 491-543.
Bjorkman, O., Badger, M. and Armond, P.A. 1978. Thermal acclimation of photosynthesis: Effect of growth temperature on photosynthetic characteristics and components of the photosynthetic apparatus in Nerium oleander. Carnegie Institution of Washington Yearbook 77: 262-276.
Bryant, J., Taylor, G. and Frehner, M. 1998. Photosynthetic acclimation to elevated CO2 is modified by source: sink balance in three component species of chalk grassland swards grown in a free air carbon dioxide enrichment (FACE) experiment. Plant, Cell and Environment 21: 159-168.
Bunce, J.A. 1998. The temperature dependence of the stimulation of photosynthesis by elevated carbon dioxide in wheat and barley. Journal of Experimental Botany 49: 1555-1561.
Ceulemans, R. and Mousseau, M. 1994. Tansley Review No. 71: Effects of elevated atmospheric CO2 on woody plants. New Phytologist 127: 425-446.
Cowling, S.A. and Sage, R.F. 1998. Interactive effects of low atmospheric CO2 and elevated temperature on growth, photosynthesis and respiration in Phaseolus vulgaris. Plant, Cell and Environment 21: 427-435.
Cure, J.D. and Acock, B. 1986. Crop responses to carbon dioxide doubling: A literature survey. Agricultural and Forest Meteorology 38: 127-145.
Cyr, H. and Pace, M.L. 1993. Magnitude and patterns of herbivory in aquatic and terrestrial ecosystems. Nature 361: 148-150.
Fernandez, M.D., Pieters, A., Donoso, C., Tezara, W., Azuke, M., Herrera, C., Rengifo, E. and Herrera, A. 1998. Effects of a natural source of very high CO2 concentration on the leaf gas exchange, xylem water potential and stomatal characteristics of plants of Spatiphylum cannifolium and Bauhinia multinervia. New Phytologist 138: 689-697.
Field, C.B., Jackson, R.B. and Mooney, H.A. 1995. Stomatal responses to increased CO2: implications from the plant to the global scale. Plant, Cell and Environment 18: 1214-1225.
Grodzinski, B., Madore, M., Shingles, R.A. and Woodrow, L. 1987. Partitioning and metabolism of photorespiratory intermediates. In: J. Biggins (Ed.), Progress in Photosynthesis Research. W. Junk, The Hague, The Netherlands, pp. 645-652.
Hakala, K. 1998. Growth and yield potential of spring wheat in a simulated changed climate with increased CO2 and higher temperature. European Journal of Agronomy 9: 41-52.
Hanson, K.R. and Peterson, R.B. 1986. Regulation of photorespiration in leaves: Evidence that the fraction of ribulose bisphosphate oxygenated is conserved and stoichiometry fluctuates. Archives of Biochemistry and Biophysics 246: 332-346.
Harley, P.C., Tenhunen. J.D. and Lange, O.L. 1986. Use of an analytical model to study the limitations on net photosynthesis in Arbutus unedo under field conditions. Oecologia 70: 393-401.
Idso, K.E. 1992. Plant Responses to Rising Levels of Carbon Dioxide: A Compilation and Analysis of the Results of a Decade of International Research into the Direct Biological Effects of Atmospheric CO2 Enrichment. Climatological Publications Scientific Paper #23, Office of Climatology, Arizona State University, Tempe, AZ.
Idso, K.E. and Idso, S.B. 1994. Plant responses to atmospheric CO2 enrichment in the face of environmental constraints: A review of the past 10 years' research. Agricultural and Forest Meteorology 69: 153-203.
Idso, S.B. 1989. Carbon dioxide, soil moisture, and future crop production. Soil Science 147: 305-307.
Idso, S.B., Kimball, B.A. and Anderson, M.G. 1985. Atmospheric CO2 enrichment of water hyacinths: Effects on transpiration and water use efficiency. Water Resources Research 21: 1787-1790.
Jurik, T.W., Webber, J.A. and Gates, D.M. 1984. Short-term effects of CO2 on gas exchange of leaves of bigtooth aspen (Populus grandidentata) in the field. Plant Physiology 75: 1022-1026.
Kimball, B.A. 1983a. Carbon dioxide and agricultural yield: An assemblage and analysis of 330 prior observations. Agronomy Journal 75: 779-788.
Kimball, B.A. 1983b. Carbon Dioxide and Agricultural Yield: An Assemblage and Analysis of 770 Prior Observations. U.S. Water Conservation Laboratory, Phoenix, AZ.
Kimball, B.A. and Idso, S.B. 1983. Increasing atmospheric CO2: effects on crop yield, water use and climate. Agricultural Water Management 7: 55-72.
Lawlor, D.W. and Mitchell, R.A.C. 1991. The effects of increasing CO2 on crop photosynthesis: A review of field studies. Plant, Cell and Environment 14: 807-818.
Long, S.P. 1991. Modification of the response of photosynthetic productivity to rising temperature by atmospheric CO2 concentrations: Has its importance been underestimated? Plant, Cell and Environment 14: 729-739.
Luscher, A., Hendrey, G.R. and Nosberger, J. 1998. Long-term responsiveness to free air CO2 enrichment of functional types, species and genotypes of plants from fertile permanent grassland. Oecologia 113: 37-45.
McMurtrie, R.E., Comins, H.N., Kirschbaum, M.U.F. and Wang, Y.-P. 1992. Modifying existing forest growth models to take account of effects of elevated CO2. Australian Journal of Botany 40: 657-677.
McMurtrie, R.E. and Wang, Y.-P. 1993. Mathematical models of the photosynthetic response of tree stands to rising CO2 concentrations and temperatures. Plant, Cell and Environment 16: 1-13.
Morison, J.I.L. 1987. Intercellular CO2 concentration and stomatal responses to CO2. In: E. Zeiger, G.D. Farquhar and I.R. Cowan (Eds.), Stomatal Function. Stanford University Press, Stanford, CA, pp. 229-251.
Mortensen, L.M. 1987. Review: CO2 enrichment in greenhouses. Crop responses. Scientia Horticulturae 33: 1-25.
Nilsen, S., Hovland, K., Dons, C. and Sletten, S.P. 1983. Effect of CO2 enrichment on photosynthesis, growth and yield of tomato. Scientia Horticulturae 20: 1-14.
Overdieck, D. and Forstreuter, M. 1994. Evapotranspiration of beech stands and transpiration of beech leaves subject to atmospheric CO2 enrichment. Tree Physiology 14: 997-1003.
Poorter, H. 1993. Interspecific variation in the growth response of plants to an elevated and ambient CO2 concentration. Vegetatio 104/105: 77-97.
Reddy, K.R., Robana, R.R., Hodges, H.F., Liu, X.J. and McKinion, J.M. 1998. Interactions of CO2 enrichment and temperature on cotton growth and leaf characteristics. Environmental and Experimental Botany 39: 117-129.
Rey, A. and Jarvis, P.G. 1998. Long-term photosynthetic acclimation to increased atmospheric CO2 concentration in young birch (Betula pendula) trees. Tree Physiology 18: 441-450.
Rogers, H.H., Bingham, G.E., Cure, J.D., Smith, J.M. and Surano, K.A. 1983. Responses of selected plant species to elevated carbon dioxide in the field. Journal of Environmental Quality 12: 569-574.
Seeman, J.R., Berry, J.A. and Downton, J.S. 1984. Photosynthetic response and adaptation to high temperature in desert plants. A comparison of gas exchange and fluorescence methods for studies of thermal tolerance. Plant Physiology 75: 364-368.
Sgherri, C.L.M., Quartacci, M.F., Menconi, M., Raschi, A. and Navari-Izzo, F. 1998. Interactions between drought and elevated CO2 on alfalfa plants. Journal of Plant Physiology 152: 118-124.
Stuhlfauth, T. and Fock, H.P. 1990. Effect of whole season CO2 enrichment on the cultivation of a medicinal plant, Digitalis lanata. Journal of Agronomy and Crop Science 164: 168-173.
Taylor, K. and Potvin, C. 1998. Understanding the long-term effect CO2 enrichment on a pasture: the importance of disturbance. Canadian Journal of Botany 75: 1621-1627.
Tognetti, R., Johnson, J.D., Michelozzi, M. and Raschi, A. 1998. Response of foliar metabolism in mature trees of Quercus pubescens and Quercus ilex to long-term elevated CO2. Environmental and Experimental Botany 39: 233-245.
Valle, R., Mishoe, J.W., Jones, J.W. and Allen Jr., L.H. 1985. Transpiration rate and water use efficiency of soybean leaves adapted to different CO2 environments. Crop Science 25: 477-482.
Vu, J.C.V., Allen Jr., L.H., Boote, K.J. and Bowes, G. 1997. Effects of elevated CO2 and temperature on photosynthesis and Rubisco in rice and soybean. Plant, Cell and Environment 20: 68-76.
Wullschleger, S.D., Post, W.M. and King, A.W. 1995. On the potential for a CO2 fertilization effect in forests: Estimates of the biotic growth factor based on 58 controlled-exposure studies. In: A.W. Woodwell and F.T. Mackensie (Eds.), Biospheric Feedbacks in the Global Climate System: Will Warming Feed the Warming? Oxford University Press, London, UK, pp. 85-107.
Wullschleger, S.D., Norby, R.J. and Gunderson, C.A. 1997. Forest trees and their response to atmospheric CO2 enrichment: A compilation of results. In: L.H. Allen, Jr., M.B. Kirkham, D.M. Olszyk and C.E. Whitman (Eds.), Advances in Carbon Dioxide Effects Research. American Society of Agronomy, Madison, pp. 79-100.




