It has been predicted that rates of coral calcification, as well as the photosynthetic rates of their symbiotic algae, will dramatically decline in response to what is typically referred to as an acidification of the world's oceans, as the atmosphere's CO2 concentration continues to rise in the years, decades, and centuries to come. As ever more pertinent evidence accumulates, however, the true story appears to be just the opposite. This summary examines such evidence obtained from field-based studies conducted in the natural ocean.
Field studies hold an advantage over laboratory-based studies in that they more aptly represent conditions in the real world, many of which conditions are impossible or impractical to incorporate into a laboratory setting. Because of this, the findings produced in field studies tend to hold more weight and help to establish greater clarity on a scientific topic or question under investigation than findings produced in a laboratory setting. Such is the case with ocean acidification. Whereas positive, negative and neutral effects from this phenomenon have been observed on corals in the laboratory setting, field-based studies in the ocean reveal the situation is much less dire than the IPCC predicts, with many studies suggesting a modest decline in oceanic pH may even favor coral calcification and growth.
Pelejero et al. (2005), for example, developed a reconstruction of seawater pH spanning the period 1708-1988, based on the boron isotopic composition (ð11B) of a long-lived massive coral (Porites) from Flinders Reef in the western Coral Sea of the southwestern Pacific. Results indicated that "there [was] no notable trend toward lower ð11B values" over the 300-year period investigated. Instead, they say that "the dominant feature of the coral ð11B record is a clear interdecadal oscillation of pH, with ð11B values ranging between 23 and 25 per mil (7.9 and 8.2 pH units)," which "is synchronous with the Interdecadal Pacific Oscillation." Furthermore, they calculated changes in aragonite saturation state from the Flinders pH record that varied between ~3 and 4.5, which values encompass "the lower and upper limits of aragonite saturation state within which corals can survive." Despite this fact, they report that "skeletal extension and calcification rates for the Flinders Reef coral fall within the normal range for Porites and are not correlated with aragonite saturation state or pH." Thus, contrary to claims of the sensitivity of coral calcification rate to changes in pH and aragonite saturation state, they found that huge cyclical changes in these parameters had essentially no detectable effect on either coral calcification or skeletal extension rates.
In a study of historical calcification rates determined from coral cores retrieved from 35 sites on the Great Barrier Reef, Lough and Barnes (1997) observed a statistically significant correlation between coral calcification rate and local water temperature, such that a 1°C increase in mean annual water temperature increased mean annual coral calcification rate by about 3.5 percent. Nevertheless, they report there were "declines in calcification in Porites on the Great Barrier Reef over recent decades." They are quick to point out, however, that their data depict several extended periods of time when coral growth rates were either above or below the long-term mean, cautioning that "it would be unwise to rely on short-term values (say averages over less than 30 years) to assess mean conditions."
As an example of this fact, they report that "a decline in calcification equivalent to the recent decline occurred earlier this century and much greater declines occurred in the 18th and 19th centuries," long before anthropogenic CO2 emissions made much of an impact on the air's CO2 concentration. Over the entire expanse of their dataset, Lough and Barnes say "the 20th century has witnessed the second highest period of above average calcification in the past 237 years," which is not exactly to be expected in light of (1) how dangerous high water temperatures are often said to be for corals, (2) the claim that Earth is currently warmer than it has been at any other time during the entire past millennium, and (3) the fact that the air's CO2 content is currently much higher than it has been for far longer than a mere thousand years.
Similar findings have been reported by Bessat and Buigues (2001), who derived a history of coral calcification rates from a core extracted from a massive Porites coral head on the French Polynesian island of Moorea that covered the period 1801-1990. They performed this work, they say, because "recent coral-growth models highlight the enhanced greenhouse effect on the decrease of calcification rate," and rather than relying on theoretical calculations, they wanted to work with real-world data, stating that the records preserved in ancient corals "may provide information about long-term variability in the performance of coral reefs, allowing unnatural changes to be distinguished from natural variability."
Bessat and Buigues found that a 1°C increase in water temperature increased coral calcification rates at the site they studied by 4.5 percent. Then, they found that "instead of a 6-14% decline in calcification over the past 100 years computed by the Kleypas group, the calcification has increased, in accordance with [the results of] Australian scientists Lough and Barnes." They also observed patterns of "jumps or stages" in the record, which were characterized by an increase in the annual rate of calcification, particularly at the beginning of the past century "and in a more marked way around 1940, 1960 and 1976," stating once again that their results "do not confirm those predicted by the Kleypas et al. (1999) model."
Another major blow to the Kleypas et al. model was provided by the work of Lough and Barnes (2000), who assembled and analyzed the calcification characteristics of 245 similar-sized massive colonies of Porites corals obtained from 29 reef sites located along the length, and across the breadth, of Australia's Great Barrier Reef (GBR), which data spanned a latitudinal range of approximately 9° and an annual average sea surface temperature (SST) range of 25-27°C. To these data they added other published data from the Hawaiian Archipelago (Grigg, 1981, 1997) and Phuket, Thailand (Scoffin et al., 1992), thereby extending the latitudinal range of the expanded dataset to 20° and the annual average SST range to 23-29°C.
This analysis revealed that the GBR calcification data were linearly related to the average annual SST data, such that "a 1°C rise in average annual SST increased average annual calcification by 0.39 g cm-2 year-1." Results were much the same for the extended dataset; Lough and Barnes report that "the regression equation [calcification = 0.33(SST) - 7.07] explained 83.6% of the variance in average annual calcification (F = 213.59, p less than 0.00)," noting that "this equation provides for a change in calcification rate of 0.33 g cm-2 year-1 for each 1°C change in average annual SST."
With respect to the significance of their findings, Lough and Barnes say they "allow assessment of possible impacts of global climate change on coral reef ecosystems," and between the two 50-year periods 1880-1929 and 1930-1979, they calculate a calcification increase of 0.06 g cm-2 year-1, noting that "this increase of ~4% in calcification rate conflicts with the estimated decrease in coral calcification rate of 6-14% over the same time period suggested by Kleypas et al. (1999) as a response to changes in ocean chemistry." Even more stunning is their observation that between the two 20-year periods 1903-1922 and 1979-1998, "the SST-associated increase in calcification is estimated to be less than 5% in the northern GBR, ~12% in the central GBR, ~20% in the southern GBR and to increase dramatically (up to ~50%) to the south of the GBR."
In light of these real-world observations, and in stark contrast to the prognostications of the IPCC, Lough and Barnes conclude that coral calcification rates "may have already significantly increased along the GBR in response to global climate change."
In another study, Carricart-Ganivet (2004) developed relationships between coral calcification rate and annual average SST based on data collected from colonies of the reef-building coral Montastraea annularis at 12 localities in the Gulf of Mexico and the Caribbean Sea, finding that calcification rate in the Gulf of Mexico increased 0.55 g cm-2 year-1 for each 1°C increase, while in the Caribbean Sea it increased 0.58 g cm-2 year-1 for each 1°C increase. Pooling these data with those of M. annularis and M. faveolata growing to a depth of 10 m at Carrie Bow Cay, Belize, those from reefs at St. Croix in the US Virgin Islands, and those of M. faveolata growing to a depth of 10 m at Curacao, Antilles, Carricart-Ganivet reports he obtained a mean increase in calcification rate of ~0.5 g cm-2 year-1 for each 1°C increase in annual average SST, which is even greater than what was found by Lough and Barnes for Porites corals.
Working at two reef sites on the northwest coast of Cuba -- one in the Guanahacabibes Gulf just off the Pinar del Rio Province and the other north of Havana Bay -- Carricart-Ganivet and Gonzalez-Diaz (2009) measured yearly coral extension rates and densities of the dominant Caribbean reef-building coral Montastraea annularis for the period 1991 to 2003, from which data they calculated annual coral calcification rates. They then plotted their results against mean annual sea surface temperature (SST, obtained from the UK's Hadley Centre) and compared their results with the earlier study of Carricart-Ganivet (2004). The results of these two investigations are presented in Figure 1, where it can be seen that they are totally compatible with each other.
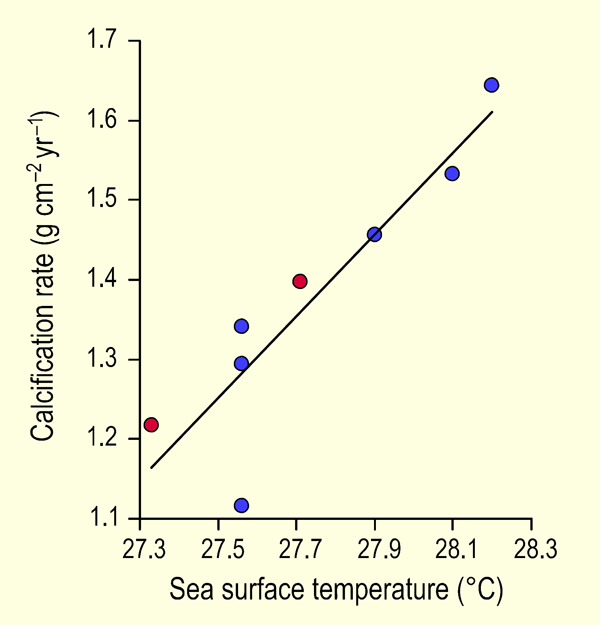
Figure 1. Mean yearly calcification rate of Montastraea annularis vs. mean annual sea surface temperature for the several sites studied by Carricart-Ganivet (2004) (blue circles) and the two sites studied by the authors (red circles). The line that has been fit to the data is described by: Calcification Rate = 0.51 SST - 12.85 (r2 = 0.82, p < 0.002). Adapted from Carricart-Ganivet and Gonzalez-Diaz (2009).
In a study devoted to corals that involves a much longer period of time, Crabbe et al. (2006) determined the original growth rates of long-dead Quaternary corals found in limestone deposits on islands in the Wakatobi Marine National Park of Indonesia, after which they compared them to the growth rates of present-day corals of the same genera living in the same area. This work revealed that the Quaternary corals grew "in a comparable environment to modern reefs"-- except, of course, for the air's CO2 concentration, which is currently higher than it has been at any other time throughout the Quaternary, which spans the past 1.8 million years. Most interestingly, therefore, their measurements indicated that the radial growth rates of the modern corals were 31 percent greater than those of their ancient predecessors in the case of Porites species, and 34 percent greater in the case of Favites species.
In a review paper, Cohen and Holcomb (2009) highlight several basic facts about the process of calcification in zooxanthellate corals. First of all, Cohen and Holcomb report what is perhaps the most fundamental fact of all, i.e., that "calcification is an active, physiological process that requires significant amounts of energy to drive it." Second, they note that "increased photosynthesis [of coral symbiotic zooxanthellae] means increased photosynthate and more energy for calcification." Third, they say that Atkinson et al. (1995) have shown that "nutritionally replete zooxanthellate corals in naturally low [aragonite] saturation-state seawaters are capable of accreting skeletons at rates comparable to those achieved by conspecifics in high-saturation-state seawaters." Fourth, the two researchers report that "today, several reefs, including Galapagos, areas of Pacific Panama, and Jarvis (southern Line Islands), experience levels of aragonite saturation equivalent to that predicted for the open ocean under two times and three times pre-industrial CO2 levels (Manzello et al., 2008; Kathryn Shamberger [PMEL/NOAA] and colleagues, pers. comm., August 2009)," and that "available data on coral colony growth rates on these reefs, albeit limited, suggest that they are equivalent to and sometimes even rival those of conspecifics in areas where aragonite saturation states are naturally high, such as the western Pacific warm pool."
Probably the most important deduction to flow from these observations is the observable fact, in the words of Cohen and Holcomb, that "naturally elevated levels of inorganic nutrients and, consequently, high levels of primary and secondary production, may already be facilitating high coral calcification rates in regions with naturally high dissolved CO2 levels," which further suggests that Earth's corals, with their genetically-diverse symbiotic zooxanthellae, are likely well equipped to deal successfully with whatever increase in the air's CO2 content will ultimately result from the burning of fossil fuels before other energy sources become viable means of providing the bulk of the energy needs of the planet's human populace.
Another good reason for not believing that the ongoing rise in the air's CO2 content will lead to reduced rates of calcification in the world's coral reefs, is that the same phenomenon that powers the twin processes of coral calcification and phytoplanktonic growth (photosynthesis) tends to increase the pH of marine waters (Gnaiger et al., 1978; Santhanam et al., 1994; Brussaard et al., 1996; Lindholm and Nummelin, 1999; Macedo et al., 2001; Hansen, 2002). This phenomenon has been shown to have the ability to dramatically increase the pH of marine bays, lagoons, and tidal pools (Gnaiger et al., 1978; Macedo et al., 2001; Hansen, 2002) as well as to significantly enhance the surface water pH of areas as large as the North Sea (Brussaard et al., 1996).
In one example of such phenomenon, Middelboe and Hansen (2007) studied the pH of a wave-exposed boulder reef in Aalsgaarde on the northern coast of Zealand, Denmark, and a sheltered shallow-water area in Kildebakkerne in the estuary Roskilde Fjord, Denmark, reporting that, in line with what would be expected if photosynthesis tends to increase surface-water pH, (1) "daytime pH was significantly higher in spring, summer and autumn than in winter at both study sites," often reaching values of 9 or more during peak summer growth periods vs. 8 or less in winter, (2) "diurnal measurements at the most exposed site showed significantly higher pH during the day than during the night," reaching values that sometimes exceeded 9 during daylight hours but that typically dipped below 8 at night, and (3) that "diurnal variations were largest in the shallow water and decreased with increasing water depth."
In addition to their own findings, Middelboe and Hansen cite those of Pearson et al. (1998), who found that pH averaged about 9 during the summer in populations of Fucus vesiculosus in the Baltic Sea; Menendez et al. (2001), who found that maximum pH was 9 to 9.5 in dense floating macroalgae in a brackish coastal lagoon in the Ebro River Delta; and Bjork et al. (2004), who found pH values as high as 9.8 to 10.1 in isolated rock pools in Sweden. Noting that "pH in the sea is usually considered to be stable at around 8 to 8.2," the two Danish researchers thus concluded that "pH is higher in natural shallow-water habitats than previously thought."
In a study investigating another potentially negative aspect of ocean acidification on corals, writing as background for their work, Meron et al. (2012) state that ocean acidification "has the potential to cause significant perturbations to the physiology of ocean organisms, particularly those such as corals that build their skeletons/shells from calcium carbonate," and they say that this phenomenon "could also have an impact on the coral microbial community, and thus may affect coral physiology and health." However, they note that most studies of declining pH effects on corals and/or their associated microbiota have typically been done under "controlled laboratory conditions," which approach clearly ignores any impacts declining pH values may have on the coral holobiont, some of which may be negative and some positive, which in the latter case is referred to as the probiotic hypothesis, as per Reshef et al. (2006).
Against this backdrop, and departing from this common protocol, the six scientists took advantage of a natural pH gradient off the coast of Ischia (Gulf of Naples, Italy), which is created by an underwater CO2 flux from volcanic vents (Hall-Spencer et al., 2008). Their purpose was to examine the potential impacts of a range of pH conditions (7.3 to 8.1) on coral microbial communities living under natural real-world conditions, focusing on two Mediterranean coral species: Balanophyllia europaea and Cladocora caespitosa.
In describing their findings, the research team reports that "pH did not have a significant impact on the composition of associated microbial communities in both coral species." They also note that "corals present at the lower pH sites exhibited only minor physiological changes," and that "no microbial pathogens were detected." Thus, they conclude that "at least for these two coral species, reduced pH does not seem to significantly reduce coral health," which further suggests that some of the contrary results obtained in laboratory studies could be due to the fact that "laboratory environments cannot mimic the dynamism and microbial diversity present in nature," as well as the possibility that "aquarium conditions themselves contribute to stress or disturbance in the microbial community," which view is supported in part by the finding of Kooperman et al. (2007) that "the same coral species has different associated microbial communities in the laboratory compared with field conditions."
In another study conducted in the real world of nature that challenges IPCC projections of ocean acidification, Shamberger et al. (2011) deployed newly designed "autosamplers" to collect water samples from the barrier coral reef of Kaneohe Bay, Oahu, Hawaii, every two hours for six 48-hour periods, two each in June 2008, August 2009 and January/February 2010. And based on these seawater measurements, they calculated net ecosystem calcification (NEC) and net photosynthesis (NP) rates for these periods. As expected, the six scientists found that "daily NEC was strongly negatively correlated with average daily pCO2, which ranged from 421 to 622 ppm." Most interestingly, however, they report that "daily NEC of the Kaneohe Bay barrier reef is similar to or higher than daily NEC measured on other coral reefs, even though Ωarag levels (mean Ωarag = 2.85) are some of the lowest measured in coral reef ecosystems." Shamberger et al. conclude the report of their study by saying "it appears that while calcification rate and Ωarag are correlated within a single coral reef ecosystem," as in the case of the barrier reef of Kaneohe Bay, "this relationship does not necessarily hold between different coral reef systems," and they state that it can thus be expected that "ocean acidification will not affect coral reefs uniformly and that some may be more sensitive to increasing pCO2 levels than others," which also means (taking a more positive view of the subject) that some may be less sensitive to increasing pCO2 than others.
Writing as background for their work, McCulloch et al. (2012) state that "for cold-water corals, which are already living at low levels of carbonate saturation, the shoaling of the saturation horizon as carbonate saturation states decrease [in response, for example, to rising atmospheric CO2 concentrations] has the potential to cause dramatic declines in rates of calcification, or the dissolution of the carbonate skeletons of those living at or close to the saturation horizon." But since these corals are indeed living there, they speculate that "they may have evolved adaptive strategies to counter the effects of low carbonate saturation states," one of which is to up-regulate their internal pH to a value that allows calcification to occur.
To further explore this suspected phenomenon, McCulloch et al. extended the novel approach taken by Trotter et al. (2011), based on boron isotopic systematics, to determine the relationship between seawater pH and the internal (extracellular) pHcf at the site of calcification for several azooxanthellate cold-water scleractinian corals, which were collected from a large range of depths and geographically disparate sites, including southeast Australia, Chile's Comau Fjord, the Marmara Sea, a number of sites in the Mediterranean Sea, the northeast Atlantic Ocean and the northwestern Hawaiian Islands.
The suite of "aragonitic cold-water coral species," as the eleven researchers describe them, "collectively show an overall trend of higher &Delta pH [= pHcf - seawater pH] values that is anti-correlated with seawater pH, with systematics generally consistent with biologically controlled pH up-regulation." And this result indicates that, "like symbiont-bearing tropical corals (Trotter et al., 2011), they have the ability to ameliorate or buffer external changes in seawater pH by up-regulating their pHcf at the site of calcification."
In light of these several observations, McCulloch et al. conclude that "cold-water corals are likely to be much more resilient to decreasing seawater pH from ocean acidification than previously realized," because, as they see it, "decreasing seawater pH alone will only marginally affect calcification rates since this process would be largely countered by pHcf up-regulation in cold-water corals, together with enhanced calcification rates from warming of the deep oceans." Once again, therefore, life appears to be well prepared for another environmental contingency.
Introducing their work, Thresher et al. (2011) state that concerns about ocean acidification impacting marine ecosystems "are based primarily on modeling studies and short-term laboratory exposure to low-carbonate conditions," citing Riegl et al. (2009), Veron et al. (2009) and Ries et al. (2010). And they say that "their relevance to long-term exposure in the field and the potential for ecological or evolutionary adjustment are uncertain," citing Maynard et al. (2008). Thus, in an effort "to determine the sensitivity of corals and allied taxa to long-term exposure to very low carbonate concentrations," Thresher et al. examined "the depth distribution and life-history characteristics of corals and other shell-forming megabenthos along the slopes of deep-sea seamounts and associated structure in the SW Pacific," where the gradient of water chemistry ranged from super-saturated with respect to aragonite and high-magnesium calcite (HMC) to under-saturated, even with respect to calcite.
Results of the analysis led the five researchers to report that they "found little evidence that carbonate under-saturation to at least -30% affected the distribution, skeletal composition, or growth rates of corals and other megabenthos on Tasmanian seamounts." In fact, they found that "both solitary scleractinian corals and colonial gorgonians were abundant at depths well below their respective saturation horizons and appeared healthy," while HMC echinoderms were common to as deep as they sampled (4011 m), in water that was approximately 45% under-saturated. They also report that "for both anthozoan and non-anthozoan taxa, there was no obvious difference in species' maximum observed depths as a function of skeletal mineralogy." In other words, the community "was not obviously shifted towards taxa with either less soluble or no skeletal structure at increasing depth." And in light of these observations, they write that "it is not obvious from our data that carbonate saturation state and skeletal mineralogy have any effect on species' depth distributions to the maximum depth sampled," and they say that they also saw "little evidence of an effect of carbonate under-saturation on growth rates and skeletal features."
Commenting further on their findings, Thresher et al. write that "the observation that the distributions of deep-sea corals are not constrained by carbonate levels below saturation is broadly supported by the literature," noting that "solitary scleractinians have been reported as deep as 6 km (Fautin et al., 2009) and isidid gorgonians as deep as 4 km (Roark et al., 2005)." And they say that their own data also "provide no indication that conditions below saturation per se dictate any overall shifts in community composition."
As for why things were as they observed them to be, the researchers note, as highlighted by Cohen and Holcomb (2009), that one or more cell membranes may envelope the organisms' skeletons, largely isolating the calcification process and its associated chemistry from the bulk seawater, citing the studies of McConnaughey (1989), Adkins et al. (2003) and Cohen and McConnaughey (2003), which phenomenon could presumably protect "the skeleton itself from the threat of low carbonate dissolution." In addition, they note that "calcification is energetically expensive, consuming up to 30% of the coral's available resources, and that normal calcification rates can be sustained in relatively low-carbonate environments under elevated feeding or nutrient regimes," as described in detail by Cohen and Holcomb (2009), stating that the likelihood that "elevated food availability could compensate for the higher costs of calcification in heterotrophic deep-sea species appears plausible."
Whatever the reason or reasons for the various observations of Thresher et al., their data clearly suggest, as they describe it, that "a change in carbonate saturation horizons per se as a result of ocean acidification is likely to have only a slight effect on most of the live deep-sea biogenic calcifiers," which is a most reassuring result.
Working in the Comau fjord, in March of 2010 and February and March of 2011, Jantzen et al. (2013) measured water profiles with a CTD multi-probe (conductivity, temperature, depth) profiler (plus an oxygen probe in 2010 only) along the course of the fjord, extending down to 50-60 meters in 2010 and down to 225 meters in 2011, while also collecting water samples using Niskin bottles. And in doing so, they were able to detect and describe the spatial distribution of the cold-water coral Desmophyllum dianthus that grows along the course of the fjord over its entire pH gradient, based on data that were acquired via SCUBA diving in 2004, 2005, 2007 and 2011, or by means of video transects obtained by a remotely-operated vehicle in 2004, 2005 and 2007.
From this host of data, the seven scientists determined that the cold-water coral D. dianthus grows along the course of the fjord and its entire pH range," where "it occurs in shallow depths (below 12 m, pH 8.1) as part of a deep-water emergence community, but also in [water of] 225 m depth at a pH of 7.4." Indeed, they say that it thrives close to the aragonite saturation horizon and even below it, where they found "flourishing coral banks."
In discussing their findings within a wider context, Jantzen et al. note that several other recent studies "question reduced calcification rates of corals in environments with lowered aragonite saturation state (Ωarg)," citing Marubini et al. (2008) and Jury et al. (2010), while noting that "very recent studies hint at a higher acclimatization potential of cold-water corals to ocean acidification," citing Rodolfo-Metalpa et al. (2010), Trotter et al. (2011), Form and Riebesel (2012) and McCulloch et al. (2012a,b). And their study suggests much the same thing.
Anthony et al. (2011) used "a carbon flux model for photosynthesis, respiration, calcification and dissolution coupled with Lagrangian transport to examine how key groups of calcifiers (zooxanthellate corals) and primary producers (macroalgae) on coral reefs contribute to changes in the seawater carbonate system as a function of water residence time." This work revealed, in their words, that "the carbon fluxes of corals and macroalgae drive Ωa in opposing directions," such that "areas dominated by corals elevate pCO2 and reduce Ωa, thereby compounding ocean acidification effects in downstream habitats, whereas algal beds draw CO2 down and elevate Ωa, potentially offsetting ocean acidification impacts at the local scale." And they also report that simulations for two significantly elevated CO2 scenarios (600 and 900 ppm CO2) suggested that "a shift in reef community composition from coral to algal dominance in upstream areas under ocean acidification will potentially improve conditions for calcification in downstream areas."
Field validation of the simulations of Anthony et al. was provided by Kleypas et al. (2011), who examined the roles of three key members of benthic reef communities (corals, macroalgae and sand) in modifying the chemistry of open-ocean source water, finding that "the drawdown of total dissolved inorganic carbon due to photosynthesis and calcification of reef communities can exceed the drawdown of total alkalinity due to calcification of corals and calcifying algae, leading to a net increase in aragonite saturation state." In addition, they note that there were no seagrasses on the reef flat they studied; and they state that "research suggests that seagrasses may have an additional impact on reef seawater chemistry because they enhance the alkalinity flux from sediments (Burdige and Zimmerman, 2002), and they respond to CO2 fertilization (Palacios and Zimmerman, 2007)."
In light of these several observations, it might logically be expected that reef communities would gradually alter their spatial compositions in a CO2-acreting world to the point where seagrasses and other macroalgae take up residence in upstream regions, while corals and other calcifying organisms lay claim to downstream regions. Therefore, as Anthony et al. (2011) conclude, "although the carbon fluxes of benthic reef communities cannot significantly counter changes in carbon chemistry at the scale of oceans, they provide a significant mechanism of buffering ocean acidification impacts at the scale of habitat to reef."
In providing some context for their work, Smith et al. (2013) state that "benthic marine primary producers affect the chemistry of their surrounding environment through metabolic processes." And they note, in this regard, that "photosynthesis and respiration will elevate or depress the concentration of oxygen in the diffusive boundary layer," while "acid-base regulation and biomineralization/dissolution for calcifying species can alter the relative concentration of inorganic carbon species and thus pH."
With the goal of comparing species-specific rates of change in pH and oxygen concentrations over a diel cycle for several species of common benthic coral reef organisms - including corals, turf algae, and fleshy and calcifying macroalgae - Smith et al. conducted similar studies in both the Caribbean and Pacific to assess the generality of results across divergent types of reefs. In doing so the four researchers determined that "more productive fleshy taxa have the potential to raise both oxygen and pH during the day to a greater extent than calcified species," which led them to state that their study, as well as the studies of Anthony et al. (2011) and Kleypas et al. (2011), thus suggest that "non-calcifying primary producers, especially those driving large amplitudes in diurnal pH fluctuations, may be important 'buffer organisms' against potential ocean acidification on coral reefs." The implications of such, according to Smith et al., are that "while particular species of macroalgae can negatively affect corals in a variety of ways," their study suggests that "some fleshy taxa may provide a buffering capacity to future ocean acidification scenarios," which suggests that sometime enemies can sometimes be friends!
In a discussion of the same topic, Manzello et al. (2012) write that although many people expect future ocean acidification (OA) due to rising atmospheric CO2 concentrations to reduce the calcification rates of marine organisms, we have little understanding of how OA will manifest itself within dynamic, real-world systems, because, as they correctly note, "natural CO2, alkalinity, and salinity gradients can significantly alter local carbonate chemistry, and thereby create a range of susceptibility for different ecosystems to OA." Against this backdrop, in an effort "to determine if photosynthetic CO2 uptake associated with seagrass beds has the potential to create OA refugia," as they describe it, Manzello et al. repeatedly measured carbonate chemistry across an inshore-to-offshore gradient in the upper, middle and lower Florida Reef Tract over a two-year period.
During times of heightened oceanic vegetative productivity, the five U.S. researchers found "there is a net uptake of total CO2 which increases aragonite saturation state (Ωarag) values on inshore patch reefs of the upper Florida Reef Tract," and they say that "these waters can exhibit greater Ωarag than what has been modeled for the tropical surface ocean during preindustrial times, with mean Ωarag values in spring equaling 4.69 +/- 0.10." At the same time, however, they report that Ωarag values on offshore reefs "generally represent oceanic carbonate chemistries consistent with present day tropical surface ocean conditions."
Manzello et al. hypothesize that the pattern described above "is caused by the photosynthetic uptake of total CO2 mainly by seagrasses and, to a lesser extent, macroalgae in the inshore waters of the Florida Reef Tract." And they therefore conclude that these inshore reef habitats are "potential acidification refugia that are defined not only in a spatial sense, but also in time, coinciding with seasonal productivity dynamics," which further implies that "coral reefs located within or immediately downstream of seagrass beds may find refuge from ocean acidification." And in further support of this conclusion, they cite the work of Palacios and Zimmerman (2007), which they describe as indicating that "seagrasses exposed to high-CO2 conditions for one year had increased reproduction, rhizome biomass, and vegetative growth of new shoots, which could represent a potential positive feedback to their ability to serve as ocean acidification refugia."
In one final study, Noonan et al. (2013) write that "ocean acidification (OA) is expected to negatively affect coral reefs," but they say that "little is known about how OA will change the coral-algal symbiosis on which reefs ultimately depend." In fact, they indicate that "to date it remains unknown if corals are able to respond to rising CO2 concentrations by changing to better adapted dominant Symbiodinium types after long-term exposure to elevated pCO2 in the field," where field, of course, to them means ocean. Against this backdrop Noonan et al. investigated "the dominant types of Symbiodinium associating with six species of scleractinian coral that were exposed to elevated partial pressures of carbon dioxide (pCO2) in situ from settlement and throughout their lives." This was done "at three naturally occurring volcanic CO2 seeps (pCO2 ~500 to 900 ppm, pHTotal 7.8-7.9) and adjacent control areas (pCO2 ~390 ppm, pHTotal ~8.0-8.05) in Papua New Guinea," while "Symbiodinium associated with corals living in an extreme seep site (pCO2 >1000 ppm) were also examined."
The three Australian researchers report that within five of the six species studied, "85-95% of samples exhibited the same Symbiodinium type across all sites, with remaining rare types having no patterns attributable to CO2 exposure." The sixth species of coral, however, did display "site specific differences in Symbiodinium types," but these were "unrelated to CO2 exposure." Last of all, they found that "Symbiodinium types from the coral inhabiting the extreme CO2 seep site were [also] found commonly throughout the moderate seeps and control areas." Such findings suggest that the six species of coral Noonan et al. studied, plus the various Symbiodinium types they encountered, were all able to not only survive, but to function well throughout the full range of CO2-induced pH values to which they had been exposed throughout their entire life spans.
References
Adkins, J.F., Boyle, E.A., Curry, W.B. and Lutringer, A. 2003. Stable isotopes in deep-sea corals and a new mechanism for 'vital effects'. Geochimica et Cosmochimica Acta 67: 1129-1143.
Anthony, K.R.N., Kleypas, J.A. and Gattuso, J.-P. 2011. Coral reefs modify their seawater carbon chemistry -- implications for impacts of ocean acidification. Global Change Biology 17: 3655-3666.
Atkinson, M.J., Carlson, B. and Crowe, J.B. 1995. Coral growth in high-nutrient, low pH seawater: A case study in coral growth at the Waikiki aquarium. Coral Reefs 14: 215-233.
Bessat, F. and Buigues, D. 2001. Two centuries of variation in coral growth in a massive Porites colony from Moorea (French Polynesia): a response of ocean-atmosphere variability from south central Pacific. Palaeogeography, Palaeoclimatology, Palaeoecology 175: 381-392.
Bjork, M., Axelsson, L. and Beer, S. 2004. Why is Ulva intestinalis the only macroalga inhabiting isolated rockpools along the Swedish Atlantic coast? Marine Ecology Progress Series 284: 109-116.
Brussaard, C.P.D., Gast, G.J., van Duyl, F.C. and Riegman, R. 1996. Impact of phytoplankton bloom magnitude on a pelagic microbial food web. Marine Ecology Progress Series 144: 211-221.
Burdige, D.J. and Zimmerman, R.C. 2002. Impact of sea grass density on carbonate dissolution in Bahamian sediments. Limnology and Oceanography 47: 1751-1763.
Carricart-Ganivet, J.P. 2004. Sea surface temperature and the growth of the West Atlantic reef-building coral Montastraea annularis. Journal of Experimental Marine Biology and Ecology 302: 249-260.
Carricart-Ganivet, J.P. and Gonzalez-Diaz, P. 2009. Growth characteristics of skeletons of Montastraea annularis (Cnidaria: Scleractinia) from the northwest coast of Cuba. Ciencias Marinas 35: 237-243.
Cohen, A.L. and Holcomb, M. 2009. Why corals care about ocean acidification: Uncovering the mechanism. Oceanography 22: 118-127.
Cohen, A.L. and McConnaughey, T.A. 2003. Geochemical perspectives on coral mineralization. In: Dove, P.M., Weiner, S. and de Yoreo, J.J. (Eds.). Reviews in Mineralogy and Geochemistry, Volume 54. Mineralogical Society of America, New York, New York, USA, p. 151-187.
Crabbe, M.J.C., Wilson, M.E.J. and Smith, D.J. 2006. Quaternary corals from reefs in the Wakatobi Marine National Park, SE Sulawesi, Indonesia, show similar growth rates to modern corals from the same area. Journal of Quaternary Science 21: 803-809.
Fautin, D.G., Guinotte, J.M. and Orr, J.C. 2009. Comparative depth distribution of corallimorpharians and scleractinians (Cnidaria; Anthozoa). Marine Ecology Progress Series 397: 63-70.
Form, A. and Riebesel, U. 2012. Acclimation to ocean acidification during long-term CO2 exposure in the cold-water coral Lophelia pertusa. Global Change Biology 18: 843-853.
Gnaiger, E., Gluth, G. and Weiser, W. 1978. pH fluctuations in an intertidal beach in Bermuda. Limnology and Oceanography 23: 851-857.
Grigg, R.W. 1981. Coral reef development at high latitudes in Hawaii. In: Proceedings of the Fourth International Coral Reef Symposium, Manila, Vol. 1: 687-693.
Grigg, R.W. 1997. Paleoceanography of coral reefs in the Hawaiian-Emperor Chain-revisited. Coral Reefs 16: S33-S38.
Hall-Spencer, J.M., Rodolfo-Metalpa, R., Martin, S., Ransome, E., Fine, M., Turner, S.M., Rowley, S.J., Tedesco, D. and Buia, M.-C. 2008. Volcanic carbon dioxide vents show ecosystem effects of ocean acidification. Nature 454: 96-99.
Hansen, P.J. 2002. The effect of high pH on the growth and survival of marine phytoplankton: implications for species succession. Aquatic Microbiology and Ecology 28: 279-288.
Jantzen, C., Haussermann, V., Forsterra, G., Laudien, J., Ardelan, M., Maier, S. and Richter, C. 2013. Marine Biology 160: 2597-2607.
Jury, C., Whitehead, R.F. and Szmant, A. 2010. Effects of variations in carbonate chemistry on the calcification rates of Madracis auretenra (= Madracis mirabilis sensu Wells, 1973): bicarbonate concentrations best predict calcification rates. Global Change Biology 16: 1632-1644.
Kleypas, J.A., Anthony, K.R.N. and Gattuso, J.-P. 2011. Coral reefs modify their seawater carbon chemistry -- case study from a barrier reef (Moorea, French Polynesia). Global Change Biology 17: 3667-3678.
Kleypas, J.A., Buddemeier, R.W., Archer, D., Gattuso, J-P., Langdon, C. and Opdyke, B.N. 1999. Geochemical consequences of increased atmospheric carbon dioxide on coral reefs. Science 284: 118-120.
Kooperman, N., Ben-Dov, E., Kramarsky-Winter, E., Barak, Z. and Kushmaro, A. 2007. Coral mucus-associated bacterial communities from natural and aquarium environments. FEMS Microbiology Letters 276: 106-113.
Lindholm, T. and Nummelin, C. 1999. Red tide of the dinoflagellate Heterocapsa triquetra (Dinophyta) in a ferry-mixed coastal inlet. Hydrobiologia 393: 245-251.
Lough, J.M. and Barnes, D.J. 1997. Several centuries of variation in skeletal extension, density and calcification in massive Porites colonies from the Great Barrier Reef: A proxy for seawater temperature and a background of variability against which to identify unnatural change. Journal of Experimental and Marine Biology and Ecology 211: 29-67.
Lough, J.M. and Barnes, D.J. 2000. Environmental controls on growth of the massive coral Porites. Journal of Experimental Marine Biology and Ecology 245: 225-243.
Macedo, M.F., Duarte, P., Mendes, P. and Ferreira, G. 2001. Annual variation of environmental variables, phytoplankton species composition and photosynthetic parameters in a coastal lagoon. Journal of Plankton Research 23: 719-732.
Manzello, D.P., Enochs, I.C., Melo, N., Gledhill, D.K. and Johns, E.M. 2012. Ocean acidification refugia of the Florida Reef Tract. PLoS ONE 7: e41715.
Manzello, D.P., Kleypas, J.A., Budd, D., Eakin, C.M., Glynn, P.W. and Langdon, C. 2008. Poorly cemented coral reefs of the eastern Tropical Pacific: Possible insights into reef development in a high-CO2 world. Proceedings of the National Academy of Sciences USA 105: 10.1073/pnas.0712167105.
Marubini, F., Ferrier-Pages, C., Furla, P. and Allemande, D. 2008. Coral calcification responds to seawater acidification, a working hypothesis towards a physiological mechanism. Coral Reefs 27: 491-499.
Maynard, J.A., Baird, A.H. and Pratchett, M.S. 2008. Revisiting the Cassandra syndrome: the changing climate of coral reef research. Coral Reefs 27: 745-749.
McConnaughey, T. 1989. 13C and 18O isotopic disequilibrium in biological carbonates: 1. Patterns. Geochimica et Cosmochimica Acta 53: 151-162.
McCulloch, M., Falter, J., Trotter, J. and Montagna, P. 2012a. Coral resilience to ocean acidification and global warming through pH up-regulation. Nature Climate Change 2: 1-5.
McCulloch, M., Trotter, J., Montagna, P., Falter, J., Dunbar, R., Freiwald, A., Forsterra, G., Lopez-Correa, M., Maier, C., Ruggeberg, A. and Taviani, M. 2012b. Resilience of cold-water scleractinian corals to ocean acidification: boron isotopic systematics of pH and saturation state up-regulation. Geochimica et Cosmochimica Acta 87: 21-34.
Menendez, M., Martinez, M. and Comin, F.A. 2001. A comparative study of the effect of pH and inorganic carbon resources on the photosynthesis of three floating macroalgae species of a Mediterranean coastal lagoon. Journal of Experimental Marine Biology and Ecology 256: 123-136.
Meron, D., Rodolfo-Metalpa, R., Cunning, R., Baker, A.C., Fine, M. and Banin, E. 2012. Changes in coral microbial communities in response to a natural pH gradient. ISME Journal 6: 1775-1785.
Middelboe, A.L. and Hansen, P.J. 2007. High pH in shallow-water macroalgal habitats. Marine Ecology Progress Series 338: 107-117.
Moy, A.D., Howard, W.R., Bray, S.G. and Trull, T.W. 2009. Reduced calcification in modern Southern Ocean planktonic foraminifera. Nature Geoscience 2: 276-280.
Noonan, S.H.C., Fabricius, K.E. and Humphrey, C. 2013. Symbiodinium community composition in Scleractinian corals is not affected by life-long exposure to elevated carbon dioxide. PLOS ONE 8: e63985.
Orr, J.C., Fabry, V.J., Aumont, O., Bopp, L., Doney, S.C., Feely, R.A., Gnanadesikan, A., Gruber, N., Ishida, A., Joos, F., Key, R.M., Lindsay, K., Maier-Reimer, E., Matear, R., Monfray, P., Mouchet, A., Najjar, R.G., Plattner, G.-K., Rodgers, K.B., Sabine, C.L., Sarmiento, J.L., Schlitzer, R., Slater, R.D., Totterdell, I.J., Weirig, M.-F., Yamanaka, Y. and Yool, A. 2005. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 437: 681-686.
Palacios, S. and Zimmerman, R.C. 2007. Response of eelgrass (Zostera marina L.) to CO2 enrichment: Possible impacts of climate change and potential for remediation of coastal habitats. Marine Ecology Progress Series 344: 1-13.
Pearson, G.A., Serrao, E.A. and Brawley, S.H. 1998. Control of gamete release in fucoid algae: sensing hydrodynamic conditions via carbon acquisition. Ecology 79: 1725-1739.
Pelejero, C., Calvo, E., McCulloch, M.T., Marshall, J.F., Gagan, M.K., Lough, J.M. and Opdyke, B.N. 2005. Preindustrial to modern interdecadal variability in coral reef pH. Science 309: 2204-2207.
Reshef, L, Koren, O., Loya, Y., Zilber-Rosenberg, I. and Rosenberg, E. 2006. The coral probiotic hypothesis. Applied Environmental Microbiology 8: 2067-2073.
Riegl, B., Bruckner, A., Coles, S.L., Renaud, P. and Dodge, R.E. 2009. Coral reefs: threats and conservation in an era of global change. Annals of the New York Academy of Sciences 1162: 136-186.
Ries, J.B., Cohen, A.L. and McCorkle, D.C. 2010. A nonlinear calcification response to CO2-induced ocean acidification by the coral Oculina arbuscula. Coral Reefs 29: 661-674.
Roark, E.B., Guilderson, T.P, Flood-Page, S., Dunbar, R.B., Ingram, B.L., Fallon, S.J. and McCulloch, M. 2005. Radiocarbon-based ages and growth rates of bamboo corals from the Gulf of Alaska. Geophysical Research Letters 32: 10.1029/2004GL021919.
Rodolfo-Metalpa, R., Martin, S., Ferrier-Pages, C. and Gattuso, J.P. 2010. Response of the temperate coral Cladocora caespitosa to mid- and long-term exposure to pCO2 and temperature levels projected for the year 2100 AD. Biogeosciences 7: 289-300.
Santhanam, R., Srinivasan, A., Ramadhas, V. and Devaraj, M. 1994. Impact of Trichodesmium bloom on the plankton and productivity in the Tuticorin bay, southeast coast of India. Indian Journal of Marine Science 23: 27-30.
Scoffin, T.P., Tudhope, A.W., Brown, B.E., Chansang, H. and Cheeney, R.F. 1992. Patterns and possible environmental controls of skeletogenesis of Porites lutea, South Thailand. Coral Reefs 11: 1-11.
Shamberger, K.E.F., Feely, R.A., Sabine, C.L., Atkinson, M.J., DeCarlo, E.H. and Mackenzie, F.T. 2011. Calcification and organic production on a Hawaiian coral reef. Marine Chemistry 127: 64-75.
Smith, J.E., Price, N.N., Nelson, C.E. and Haas, A.F. 2013. Coupled changes in oxygen concentration and pH caused by metabolism of benthic coral reef organisms. Marine Biology 160: 2437-2447.
Thresher, R.E., Tilbrook, B., Fallon, S., Wilson, N.C. and Adkins, J. 2011. Effects of chronic low carbonate saturation levels on the distribution, growth and skeletal chemistry of deep-sea corals and other seamount megabenthos. Marine Ecology Progress Series 442: 87-99.
Trotter, J.A., Montagna, P., McCulloch, M.T., Silenzi, S., Reynaud, S., Mortimer, G., Martin, S., Ferrier-Pages, C., Gattuso, J.-P. and Rodolfo-Metalpa, R. 2011. Quantifying the pH 'vital effect' in the temperate zooxanthellate coral Cladocora caespitosa: validation of the boron seawater pH proxy. Earth and Planetary Science Letters 303: 163-173.
Veron, J.E.N., Hoegh-Guldberg, O., Lenton, T.M., Lough, J.M., Obura, D.O., Pearce-Kelly, P., Sheppard, C.R.C., Spalding, M., Stafford-Smith, M.G. and Rogers, A.D. 2009. The coral reef crisis: The critical importance of <350 ppm CO2. Marine Pollution Bulletin 58: 1438-1436.
Last updated 9 April 2014



