As the atmosphere's CO2 concentration continues to rise, most trees will likely exhibit increased rates of photosynthesis and biomass production, which can subsequently lead to an increase in the amount of timber that will likely be required to meet the growing needs of earth's expanding human population. However, some individuals have predicted that CO2-induced global warming will counteract the growth-promoting effects of atmospheric CO2 enrichment and actually reduce tree growth. Therefore, in order to determine if this widely-trumpeted claim has any validity, we turn to the peer-reviewed scientific literature to both report and summarize the results of several CO2-enrichment studies that were designed to reveal the concurrent effects of elevated CO2 and air temperature on the growth of trees and other woody plants.
Before progressing, however, we highlight the fact that the optimum growth temperature for several plants has already been shown to rise substantially with increasing levels of atmospheric CO2 (Berry and Bjorkman, 1980; Stuhlfauth and Fock, 1990; McMurtrie et al., 1992; McMurtrie and Wang, 1993). This phenomenon is described at length by Long (1991), who calculated from well-established plant physiological principles that most C3 plants should increase their optimum growth temperature by approximately 5°C in response to a 300 ppm increase in the atmosphere's CO2 concentration. Subsequently, Cowling and Sykes (1999) produced a review of the pertinent scientific literature, demonstrating that this was indeed the case for a number of plants. Thus, one would logically expect plant photosynthetic rates to rise in tandem with concomitant increases in the air's CO2 concentration and temperature, as previously documented by Idso and Idso (1994). Hence, we here proceed to see if these positive CO2 x temperature interactions have been validated in the scientific literature of the past couple of decades for various tree species.
In the study of Kellomaki and Wang (2001), birch seedlings were grown at atmospheric CO2 concentrations of 350 and 700 ppm in combination with ambient and elevated (ambient plus 3°C) air temperatures; and after five months of treatment, they reported that the photosynthetic rates of the CO2-enriched seedlings were 21 and 28% greater than those displayed by their ambiently-grown counterparts at both ambient and elevated air temperatures, respectively. In another study, Carter et al. (2000) observed that a 300 ppm increase in the air's CO2 content allowed leaves of sugar maple seedlings to remain green and non-chlorotic when exposed to air temperatures 3°C above ambient air temperature, while seedlings fumigated with ambient air exhibited severe foliar chlorosis when exposed to the same elevated air temperatures, which results indicate that at elevated air temperatures, rates of photosynthesis are greater and foliar health is typically better in CO2-enriched as opposed to ambiently-grown trees.
Many other studies report similar results. Sheu et al. (1999), for example, grew a sub-tropical tree at day/night temperatures of 25/20 (ambient) and 30/25°C (elevated) for six months and found that seedlings exposed to 720 ppm CO2 displayed photosynthetic rates that were 20 and 40% higher, respectively, than those of their ambiently-grown controls. In addition, the CO2-induced increases in total dry weight for this species were 14 and 49%, respectively, at the ambient and elevated air temperatures. Likewise, Maherali et al. (2000) found that a 5°C increase in ambient air temperature increased the CO2-induced biomass enhancement resulting from a 750 ppm CO2 enrichment of ponderosa pine seedlings from 42 to 62%. In addition, Wayne et al. (1998) reported that a 5°C increase in the optimal growth temperature of yellow birch seedlings fumigated with an extra 400 ppm CO2 increased the CO2-induced increase in biomass from 60% to an amazing 227%. Thus, the beneficial effects of elevated CO2 on tree photosynthesis and growth is often further enhanced by elevated air temperatures, which phenomenon can also be assessed during natural seasonal temperature changes, as documented by Hymus et al. (1999) for loblolly pine and Roden et al. (1999) for snow gum seedlings.
In some cases, however, there appear to be little interactive effects between elevated CO2 and temperature on photosynthesis and growth in tree species. For example, when Tjoelker et al. (1998a) grew seedlings of quaking aspen, paper birch, tamarack, black spruce and jack pine at atmospheric CO2 concentrations of 580 ppm, they reported an average increase in photosynthetic rates of 28%, irrespective of air temperature, which varied from 18 to 30°C. And after analyzing the CO2-induced increases in dry mass for these seedlings, Tjoelker et al. (1998b) further reported that dry mass values were about 50 and 20% greater for the deciduous and coniferous species, respectively, again irrespective of air temperature.
But the list of recent studies of woody plants that experience a CO2-induced enhancement of growth in response to environmental warming goes on and on, starting with Hamerlynck et al. (2000), who grew seedlings of the evergreen perennial shrub Larrea tridentata in glasshouses maintained at atmospheric CO2 concentrations of 360, 550, and 700 ppm for one year, while half of the seedlings had water withheld from them for three months prior to a nine-day high-temperature treatment. This work revealed that elevated CO2 largely offset the detrimental effects of drought and high temperature on water relations and photosynthesis in this particular species. And averaged across the entire experiment, the photosynthetic rates of seedlings grown at 550 and 700 ppm CO2 were 31 and 90% greater, respectively, than rates displayed by the ambiently-grown control plants.
Usami et al. (2001) grew two-year-old saplings of Quercus myrsinaefolia, an evergreen broad-leaved oak species, in controlled environment chambers having various atmospheric CO2 concentrations and air temperatures for approximately one year, in order to study the interactive effects of elevated CO2 and temperature on the development and growth of this important tree, which is widely distributed throughout Laos, Vietnam, China, Taiwan, South Korea and southwestern Japan. And in doing so, they found that in ambient air, 3 and 5°C increases in air temperature boosted final sapling biomass by 53 and 47%, respectively, while at elevated CO2 concentrations that were either 1.5 or 2 times greater than the ambient CO2 concentration, the same 3 and 5°C increases in air temperature enhanced final biomass by 110 and 140%, respectively.
Turbull et al. (2002) manipulated day/night air temperatures around 4-m-tall cottonwood (Populus deltoides Bartr. Ex Marsh) trees growing within large experimental enclosures in order to study the effects of temperature on carbon relations. This work revealed that a 6°C increase in daytime temperature, coupled with a 10°C increase in nighttime temperature, enhanced rates of net photosynthesis by 64% and rates of dark respiration by a larger 77%. On an absolute scale, however, the photosynthetic carbon gains due to the daytime temperature increase were nearly an order of magnitude greater than the nocturnal carbon losses caused by the greater increase in nighttime temperature. Consequently, if the earth were to begin to warm again, carbon uptake by cottonwood trees should significantly increase.
Peltola et al. (2003) constructed closed chambers around 20-year-old Scots pine (Pinus sylvestris L.) trees growing on a low-nitrogen-containing soil; and for three years thereafter, the trees in the chambers were fumigated with air containing either 350 or 700 ppm CO2 at either ambient or elevated air temperatures (about 4°C above ambient temperatures), in order to study the effects of elevated CO2 and air temperature on stem development in this coniferous species when growing on a soil low in nitrogen. And what did they find? After three years of treatment, cumulative stem diameter growth in the CO2-enriched trees grown at ambient air temperature was 57% greater than that displayed by control trees grown at ambient CO2 and ambient air temperature. But the trees subjected to elevated CO2 and elevated air temperature exhibited cumulative stem-diameter growth that was 67% greater than that displayed by trees grown in ambient air at ambient air temperatures.
Working with Scots pine as well as Norway spruce (Picea abies (L.) Karst.), Sallas et al. (2003) grew seedlings of both species for 50 days in computer-controlled environmental growth chambers in air of ambient or twice-ambient CO2 concentration (normal or elevated (EC) treatments) at day/night temperature combinations of 19/12°C or 23/16°C (normal or elevated (ET) treatments), while making a host of measurements on them. And in doing so, they found that the seedlings of both species accumulated the most biomass in the combined EC + ET treatment.
Hymus et al. (2003) studied net ecosystem exchange (NEE) of CO2 in a scrub-oak ecosystem - 85-90% of the aboveground biomass of which was comprised of three oak species (Quercus myrtifolia, Quercus geminata and Quercus chapmanii) - on Merritt Island within NASA's Kennedy Space Center on the coast of central Florida (USA). This ecosystem was completely burned in January of 1996, after which sixteen open-top chambers (OTCs) were placed upon it in the spring of that year, half of which were maintained at the ambient atmospheric CO2 concentration while the other half were maintained at ambient plus 350 ppm, with routine measurements being started in June 1999 and continued for 25 months through July 2001. This effort revealed that (1) the extra CO2 supplied to the CO2-enriched OTCs "increased maximum NEE and the apparent quantum yield of the NEE during the photoperiod," and that (2) the magnitude of the stimulation of maximum NEE, expressed per unit ground area, "was seasonal, rising from 50% in the winter to 180% in the summer," in accord with what is known about the interactive effects of atmospheric CO2 enrichment and daily, seasonal and multi-year warming.
Turnbull et al. (2004) studied four- to five-meter-tall cottonwood trees (Populus deltoides Bartr.) that had been grown for three years in air of different CO2 concentrations (420, 800 and 1200 ppm) in the three bays of the Biosphere 2 facility near Tucson, Arizona, USA, where the trees were maintained at three different nocturnal temperatures (15, 20 or 25°C) and a single constant daytime temperature (31 +/- 1°C) in a short-term experiment in which maximum photosynthesis (Amax) rates at growth CO2 concentrations were routinely measured. This work revealed that as nocturnal air temperature rose from 15 to 25°C, subsequent daytime Amax increased by 16% in air of 420 ppm CO2, 12% in air of 800 ppm CO2 and 4% in air of 1200 ppm CO2, leading them to conclude that "at future elevated night temperatures suggested by global climate monitoring and modeling, net photosynthesis at elevated CO2 may be increased," although it appears that the response could saturate at a CO2 partial pressure somewhat in excess of 1200 ppm CO2, the latter of which values, however, is far greater than anyone is suggesting will ever be reached as a consequence of mankind's burning of fossil fuels.
But what if air temperatures get really hot, for some as yet unknown reason? In an earlier study that sheds some important light on this subject, Idso et al. (1995) grew well watered and fertilized sour orange (Citrus aurantium L.) trees from the seedling stage out-of-doors at Phoenix, Arizona (USA) in clear-plastic-wall open-top chambers that were continuously maintained at mean atmospheric CO2 concentrations of either approximately 400 or 700 ppm for 5.5 years, while during the warmest parts of some of the hottest days of summer, rates of net photosynthesis of fully-expanded outer-canopy sunlit leaves and the temperatures of those leaves were concurrently measured.
The figure below portrays the results of plotting their net photosynthesis measurements against leaf temperature. Based on the linear regression lines fit to the data, it can be determined that the 75% increase in the air's CO2 content led to a 75% enhancement of leaf net photosynthesis at a leaf temperature of 31°C, a 100% enhancement at a leaf temperature of 35°C, and a 200% enhancement at 42°C. At higher leaf temperatures, it can also be seen that the net photosynthetic rate of the foliage growing in ambient air dropped all the way to zero at 47°C (making the CO2-induced enhancement of photosynthesis at that point essentially infinite), and that it actually became negative thereafter (which condition, if prolonged, would ultimately lead to plant death).
In the CO2-enriched trees, on the other hand, the net photosynthetic rate of the foliage was still substantial at 47°C; and the regression line for those trees suggests that their mean rate of foliage net photosynthesis likely would not have declined to zero until leaf temperature reached 54°C, which is approximately 7°C above the upper-limiting temperature for positive net photosynthesis in the trees that were grown in ambient air.
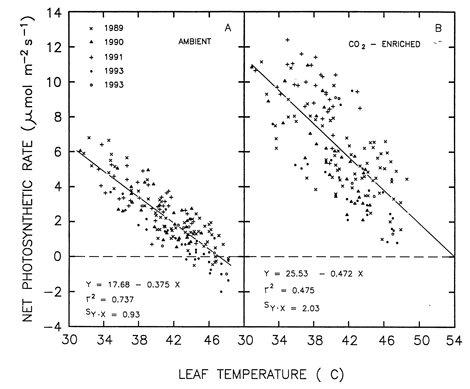
Leaf net photosynthetic rate vs. leaf temperature for the foliage of sour orange trees growing in air of either 400 or 700 ppm CO2. Adapted from Idso et al. (1995).
In light of these findings, it can be appreciated that if earth's air temperature continues to rise significantly in the future - for whatever reason - we can only hope that the air's CO2 content rises right along with it; because elevated concentrations of atmospheric CO2 are a powerful antidote for the ill - and sometimes even deadly - effects that might otherwise be experienced by much of earth's vegetation.
In a study that reaches somewhat similar conclusions, Lewis et al. (2001) grew Douglas fir (Psuedotsuga menziesii (Mirb.) Franco) seedlings in sunlit chambers programmed to track either ambient atmospheric CO2 concentration or ambient + 200 ppm CO2, as well as either ambient air temperature or ambient + 4°C, over a 21-month period, while they measured light-saturated rates of net photosynthesis at approximately monthly intervals. This work revealed that the extra CO2 they supplied to the seedlings "increased net photosynthetic rates by an average of 21% across temperature treatments during both the 1996 hydrologic year, the third year of exposure, and the 1997 hydrologic year," while "elevated mean annual temperature increased net photosynthetic rates by an average of 33% across CO2 treatments during both years." In addition, they found that "between February and August 1996, the short-term temperature optima for photosynthesis shifted by approximately 10°C higher in both CO2 treatments," and that the elevated CO2 treatment "increased the short-term (minutes to hours) temperature optima for photosynthesis, as has been observed in other tree species (Idso and Idso, 1994; Eamus et al., 1995)." And as a result of these observations, the four researchers concluded that "an increase of 200 ppm above current atmospheric CO2 concentrations may shift temperature optima upward 3-4°C, paralleling the increase in mean annual temperatures predicted to occur during the next century," and that "by shifting temperature optima upward, elevated CO2 may 'acclimate' photosynthetic processes to future temperature regimes."
More recently, Huang et al. (2007) compared, synthesized and evaluated the scientific literature to that point in time, describing (1) atmospheric CO2 enrichment experiments conducted on trees and (2) empirical tree-ring studies designed to determine if the growth-promoting effects of rising atmospheric CO2 concentrations occur in natural forests. And as a result of their analyses, they were able to report that numerous CO2-enrichment experiments have "demonstrated significantly positive physiological and growth responses of trees to CO2, providing strong evidence to support the direct CO2 fertilization effect (increased photosynthesis, water use efficiency, above- and below-ground growth) and thus allowing prediction of which ecosystems might be most responsive to CO2," with their thoughts in this regard being "warm, moderately drought-stressed ecosystems with an ample nitrogen supply," because, as they continue, "drought-stressed trees could benefit from increased water use efficiency to enhance growth." They note, however, that tree-ring studies on the cold and arid Tibetan Plateau also "showed significant growth enhancements as well as increased water use efficiency (24.7% and 33.6% for each species, respectively) in Qilian juniper and Qinghai spruce since the 1850s," citing as evidence for this statement the studies of Zhang et al. (2003), Shao et al. (2005), Liang et al. (2006), Huang and Zhang (2007) and Zhang and Qiu (2007).
One year later, Hickler et al. (2008) evaluated the process-based LPJ-GUESS model of vegetation dynamics and biogeochemistry (Smith et al., 2001; Hickler et al., 2004) via a site-by-site comparison with the results of four temperate forest FACE experiments (Norby et al., 2005). Then, after demonstrating that the model simulations adequately reproduced the magnitude of the FACE site measurements - a mean model-derived net primary productivity (NPP) increase of 25.9% for CO2 raised to a value of 550 ppm vs. a mean measured NPP increase of 27.8% for the same CO2 increase - they conducted what they called a "global forest FACE experiment" to see what the successfully-reality-tested model suggested about CO2 enrichment effects on the NPP of earth's boreal and tropical forests, as well as its temperate forests. For the world as a whole, the model suggested that raising the air's CO2 concentration to 550 ppm would increase the NPP of its temperate forests by an average of 25.7%, but that its boreal forests would only have their NPP raised by 15.1%, while its tropical forests would experience an NPP increase of 35.1%. However, Hickler et al. note that warming "is likely to increase NPP more in cold northern regions than close to the equator because of a greater proportional growing season extension in temperature-limited environments." Consequently, in the case of concomitant CO2 enrichment and warming - the latter of which is typically greater at higher latitudes - all of earth's forests might well be equally hugely benefited.
Publishing in the same year, Martinez-Vilalta et al. (2008) described how they used tree-ring data from the Catalan Ecological and Forest Inventory "to study the temporal variability of Scots pine stem radial growth (period 1901-1997) across a relatively large region (Catalonia, NE Spain) situated close to the southern limit of the distribution of the species." This inventory, in their words, "included a total of 10,664 plots randomly distributed throughout the forested area of Catalonia," where Scots pine was present in 30.2% of the plots and dominant in 18.4% of them. The results of this laborious undertaking, as they describe it, "showed an overall increase of 84% in Scots pine BAI [basal area increment] during the 20th century, consistent with most previous studies for temperate forests" and in harmony with the greening of the earth phenomenon that has accompanied the historical increase in the air's CO2 content. And in this regard, they make a point of stating that "this trend was associated with increased atmospheric CO2 concentration," which they interpret to be "a fertilization effect." Over the same time period, however, the five researchers note that "there was also a marked increase in temperature across the study region (0.19°C per decade on average)," and they note that "this warming had a negative impact on radial growth, particularly at the drier sites." But they determined that "its magnitude was not enough to counteract the fertilization effect."
Jumping ahead a couple of years, Darbah et al. (2010) measured the effects of a natural and prolonged heat wave on the photosynthetic rates of quaking aspen (Populus tremuloides Michx) and paper birch (Betula papyrifera) trees that had been grown from the seedling stage for an additional nine years in the Free-Air CO2-Enrichment (FACE) facility near Rhinelander, Wisconsin (USA), where from 0700 to 1900 hours each day throughout the growing season, half of the trees were exposed to an extra ~190 ppm of CO2. The results of the study of the heat wave event indicated that the aspen trees "showed no visible symptoms of stress," but that the birch trees exhibited "leaf curling and then yellowing of leaves and finally leaf shedding," with trees in the control treatment dropping 33% of their leaves, while trees in the CO2-enriched treatment dropped only 20%. Also, in terms of rates of photosynthesis at saturating light intensities, they found that aspen clone 42E exhibited a 30% CO2-induced increase in the 32-35°C temperature range, but a whopping 218% in the 36-39°C range. Similarly, aspen clone 271 exhibited a 38% CO2-induced increase in the 32-35°C range, but a 199% increase in the 36-39°C range. Contemporaneously, the birch trees exhibited a 95% CO2-induced increase in photosynthetic rates in the 32-35°C range, but a 297% increase in the 36-39°C range.
In discussing their findings further, the four researchers state that they are in agreement with those of Idso and Kimball (1992), who reported that elevated CO2 (ambient + 300 ppm) increased net photosynthetic rates in sour orange tree (Citrus aurantium L.) leaves exposed to full sunlight by 75, 100 and 200% compared to leaves in ambient CO2 air at temperatures of 31, 35 and 42°C, respectively, suggesting that "elevated CO2 ameliorates heat stress in tree leaves." They also note that their observations "agree with Veteli et al. (2007), who reported that elevated CO2 ameliorated the negative effects of high temperature in three deciduous tree species," and that "Wayne et al. (1998) reported that elevated CO2 ameliorated high temperature stress in yellow birch trees (Betula alleghaniensis)." Hence, they conclude that "in the face of rising atmospheric CO2 and temperature (global warming), trees will benefit from elevated CO2 through increased thermotolerance."
Contemporaneously, Ghannoum, et al. (2010b) grew individual well watered and fertilized plants of two species of Australian eucalypts - faster-growing Eucalyptus saligna and slower-growing E. sideroxylon - from seed in 10-L pots filled with 9 kg of loamy-sand in naturally-lit glasshouse compartments maintained at either ambient or ambient + 4°C air temperature and three different CO2 concentrations (280, 400 or 650 ppm) for a period of 140 days, while measuring various plant responses throughout the course of the experiment. Among other things, this protocol revealed that light-saturated net photosynthesis (Asat) increased by ~50% with each step-increase in the air's CO2 concentration - i.e., going from 280 to 400 ppm, and going from 400 to 650 ppm - and that in the higher of the two temperature treatments, the optimal temperature for Asat increased by 2-7°C across the three CO2 treatments. And they note, in this regard, that these results "partly explain the strong growth responses to elevated CO2 and temperature observed in a previous study with the same eucalypt seedlings," citing the study of Ghannoum et al. (2010a).
In introducing their study of the nature of future forests and their inhabitants, Keenan et al. (2011) indicate that climate models consistently project significant increases in temperature and decreases in precipitation in the Mediterranean basin; and they say that these changes may have a large impact on current Mediterranean forests and the related ecosystem services they provide. More particularly, they state that niche-based models - also known as bioclimatic envelope models or habitat models - are by far the most commonly used method for predicting potential species distribution responses to future climatic changes; and they say that these models typically predict significant negative consequences for terrestrial plants and animals in the face of increasing atmospheric CO2 concentration.
They, however, prefer process-based models, which describe eco-physiological processes ranging from purely empirical relationships to mechanistic descriptions based on physical laws. And they write that these models - supported by experiments and growth and yield surveys - "suggest that global warming will have a positive impact on forest productivity (van der Meer et al., 2002; Nigh et al., 2004; Norby and Luo, 2004; Briceņo-Elizondo et al., 2006; Gaucharel et al., 2008), due to the direct fertilization effect of increased CO2 and indirect effects such as lengthening of the growing period." Thus, to elucidate the difference in results obtained by employing these two approaches to divining the future, the five researchers assessed and compared the projections of each of them when applied to stands of three forest species (Quercus ilex, Pinus halepensis and Pinus sylvestris) that have widely contrasting distributions in continental Spain.
In pursuing this course of action, Keenan et al. found that CO2 fertilization through projected increased atmospheric CO2 concentrations tends to increase forest productivity in mechanistic process-based models (despite increased drought and presumed temperature stress) by up to three times that of the non-CO2 fertilization scenario by the period 2050-2080, in stark contrast to projections of reduced habitat suitability based on niche-based models for the same period. Thus, they write that their results show that "previous reports of species decline in continental Spain (e.g. Benito-Garzon et al., 2008) may be overestimated due to two reasons." One of these is the use of only one predictive niche-based model, and the other is the failure to account for positive effects of CO2 fertilization in a warming world. And they add that similar studies in other regions that do not consider these two aspects, are also potentially overestimating species decline due to climate change, noting that "niche-based model results also likely overestimate the decline in [habitat] suitability," and they therefore conclude that "an organism's niche must be modeled mechanistically if we are to fully explain distribution limits," additionally citing the work of Kearney (2006) in this regard.
In the contemporary study of Osorio et al. (2011), impacts of drought and high-temperature stresses on photosynthesis, energy partitioning and membrane lipids - as well as the potential ability of Carob or St. John's (Ceratonia siliqua) trees to attenuate oxidative damage - were investigated in seedlings growing within controlled-environment chambers, where they were rooted in 3-dm3 pots filled with a 2:1 mixture of a fertilized substrate and natural soil, and where they were maintained under two thermal regimes - low and high temperature (LT: 25/18°C; HT: 32/21°C) - and three soil water conditions (control, water stress and rewetting), while numerous physiological and biochemical plant properties and processes were repeatedly monitored. This work revealed that the decrease in net photosynthesis (PN) caused by drought was 33% in the LT chamber and 84% in the HT chamber. However, they found that "the negative effects of soil drying on PN and stomatal conductance of HT plants were no longer detected 36 hours following rewatering." And they add that "although C. siliqua seedlings exhibit clear signs of oxidative stress under drought and high temperature, they retain a remarkable ability to quickly restore normal physiological activity upon rehydration," so much so, in fact, that the five Portuguese scientists say they "can state that although C. siliqua seedlings exhibit clear signs of oxidative stress under drought and high temperature, they retain a remarkable ability to quickly restore normal physiological activity upon rehydration, which let us believe that they can satisfactorily deal with predicted climate warming and increased soil drying in the Mediterranean area."
Inching ever closer to the present, Wertin et al. (2012) examined the influence of elevated temperature (ambient + 2°C) and atmospheric CO2 concentration (700 ppm), applied singly and in combination, on biomass accumulation and the temperature response of net photosynthesis (Anet) and leaf respiration (Rd) of loblolly pine (Pinus taeda L.) seedlings grown simultaneously at a northern and a southern site within the species' range, where the long-term mean growing season temperature (from February through October) at the cool site was 15.2°C and at the warm site was 21.5°C, and where the well watered and fertilized seedlings were grown over the course of two consecutive years in half-cylindrical polyfilm-enclosed chambers located in open fields. And as a result of this effort, they determined, as they describe it, that (1) "biomass accumulation was substantially greater at the warmer site compared with the cooler site regardless of treatment," that (2) "at each site, biomass accumulation was greater in the elevated temperature treatment compared with the ambient treatment," and that (3) "elevated CO2 increased biomass accumulation and Anet at both sites and in both temperature treatments." In concluding their report, therefore, the five University of Georgia (USA) researchers state that their study "provides an indication that future projected increases in CO2 and air temperature of 700 ppm and +2°C, respectively, are likely to increase loblolly pine growth in most, if not all, of its current range." And they state that "the large number of studies that have reported an increase in tree growth in elevated growth temperatures compared with current ambient temperature (Way and Oren, 2010) suggest that other species may respond similarly."
Publishing close to the same time on this subject were Ameye et al. (2012), who wrote as background for their work that in studies where the air's CO2 content has been doubled, "increases in net photosynthesis were reported ranging from 43% to 192% in Pinus taeda (Teskey, 1997; Tissue et al., 1997; Ellsworth, 1999; Wertin et al., 2010; Frenck et al., 2011) and from 30% to 256% in Quercus rubra (Kubiske and Pregitzer, 1996; Anderson and Tomlinson, 1998; Cavender-Bares et al., 2000)." Likewise, they say that "generally, an increase in air temperature also has a positive effect on net photosynthesis and growth," citing Sage and Kubien (2007) and Way and Oren (2010). But how might loblolly pine and northern red oak trees respond to the extreme heat waves that are often predicted to occur in a future CO2-enriched world?
In their desire to answer this question, and working with the most recent fully-developed leaves of well watered and fertilized seedlings of Pinus taeda and Quercus rubra grown in 7.6-L pots out-of-doors at Athens, Georgia (USA) within polyethylene chambers maintained at ambient and elevated air temperatures (Tamb and Tamb + 3°C), as well as seven-day heat waves consisting of a biweekly +6°C heat wave or a monthly +12°C heat wave - which treatments were maintained throughout the growing season - Ameye et al. measured rates of net photosynthesis before, during and after the many mid-summer heat waves they created. In doing so, they found that "an immediate and significant decline in net photosynthesis was observed in seedlings that were subjected to a +12°C heat wave, but not in seedlings subjected to a +6°C heat wave." They also found that "after the third day of the +12°C heat wave, net photosynthesis values stabilized at positive values and did not show signs of further reduction, indicating that the photosynthetic apparatus did not accrue additional stress or damage as the heat wave continued." In light of these findings, therefore, they concluded that "if soil moisture is adequate, trees will experience negative effects in photosynthetic performance only with the occurrence of extreme heat waves." And in light of the fact that "elevated CO2 diminished these negative effects," they opine that "the future climate may not be as detrimental to plant communities as previously assumed."
In concluding this literature review, it is instructive to consider the case of the Paleocene-Eocene Thermal Maximum or PETM of some 56 million years ago, which according to Jaramillo et al. (2010), "was one of the most abrupt global warming events of the past 65 million years (Kennett and Stott, 1991; Zachos et al., 2003; Westerhold et al., 2009)." It was presumed driven, as they describe it, by "a massive release of 13C-depleted carbon (Pagani et al., 2006; Zeebe et al., 2009)" that led to "an approximate 5°C increase in mean global temperature in about 10,000 to 20,000 years (Zachos et al., 2003)." And during this period of planetary warming, they say it was thought by many that earth's tropical ecosystems "suffered extensively because mean temperatures are surmised to have exceeded the ecosystems' heat tolerance (Huber, 2008)."
But was that really so? Did the ancient warming of the world truly constitute a major problem for the planet's rainforests?
In an attempt to answer this important question, the 29 researchers, hailing from eight different countries, analyzed pollen and spore contents and the stable carbon isotopic composition of organic materials obtained from three tropical terrestrial PETM sites in eastern Colombia and western Venezuela; and this work revealed - contrary to the prevailing wisdom of the recent past - that the onset of the PETM was "concomitant with an increase in diversity produced by the addition of many taxa (with some representing new families) to the stock of preexisting Paleocene taxa." And they determined that this increase in biodiversity "was permanent and not transient."
In discussing their findings, Jaramillo et al. write that "today, most tropical rainforests are found at mean annual temperatures below 27.5°C," and they say that several scientists have argued that "higher temperatures could be deleterious to the health of tropical ecosystems," citing Stoskopf (1981), Bassow et al. (1994), Lewis et al. (2004), Huber (2008, 2009) and Tewksbury et al. (2008) in this regard. In fact, they report that tropical warming during the PETM is actually believed to have produced intolerable conditions for tropical ecosystems, citing the writings of Huber (2008, 2009). Nevertheless, they reiterate that at the sites that they studied, "tropical forests were maintained during the warmth of the PETM (~31° to 34°C)," and they thus conclude that "it is possible that higher Paleocene CO2 levels (Royer, 2010) contributed to their success."
In regard to this hypothesis, such would indeed appear to be the case, in light of what is now the well-established fact that most woody plants tend to exhibit their greatest photosynthetic rates at increasingly warmer temperatures as the air's CO2 content continues to rise, as is confirmed by the many studies of this phenomenon discussed above. And in light of Jaramillo et al.'s findings, it is becoming ever more clear that greater warmth and atmospheric CO2 concentrations are not the "twin evils" that the world's climate alarmists typically make them out to be. Quite to the contrary, they are just what earth's ecosystems need, in order to make them both more stable and more productive, which characteristics are absolutely essential for sustaining the still-increasing human population of the planet, as well as preserving what yet remains of what one could call wild nature.
In conclusion, the scientific literature of the past few decades continues to indicate that as the air's CO2 content rises, trees will likely exhibit enhanced rates of photosynthesis and biomass production that will not be negated by any global warming that might occur concurrently. In fact, if the ambient air temperature rises, the growth-promoting effects of atmospheric CO2 enrichment will likely rise right along with it. Thus, the future ability of earth's trees to produce greater amounts of biomass and, therefore, more timber products to meet the increasing needs of earth's expanding human population, looks promising indeed, just as long as the atmosphere's CO2 concentration continues to rise.
References
Ameye, M., Wertin, T.M., Bauweraerts, I., McGuire, M.A., Teskey, R.O. and Steppe, K. 2012. The effect of induced heat waves on Pinus taeda and Quercus rubra seedlings in ambient and elevated CO2 atmospheres. New Phytologist 196: 448-461.
Anderson, P.D. and Tomlinson, P.T. 1998. Ontogeny affects response of northern red oak seedlings to elevated CO2 and water stress. I. Carbon assimilation and biomass production. New Phytologist 140: 477-491.
Bassow, S.L., McConnaughay, K.D. and Bazzaz, F.A. 1994. The Response of temperate tree seedlings grown in elevated CO2 to extreme temperature events. Ecological Applications 4: 593-603.
Benito-Garzon, M., Sanchez de Dios, R. and Sainz Ollero, H. 2008. Effects of climate change on the distribution of Iberian tree species. Applied Vegetation Science 11: 169-178.
Berry, J. and Bjorkman, O. 1980. Photosynthetic response and adaptation to temperature in higher plants. Annual Review of Plant Physiology 31: 491-543.
Briceņo-Elizondo, R., Garcia-Gonzalo, J., Peltola, H., Matala, J. and Kellomaki, S. 2006. Sensitivity of growth of Scots pine, Norway spruce and silver birch to climate change and forest management in boreal conditions. Forest Ecology and Management 232: 152-167.
Carter, G.A., Bahadur, R. and Norby, R.J. 2000. Effects of elevated atmospheric CO2 and temperature on leaf optical properties in Acer saccharum. Environmental and Experimental Botany 43: 267-273.
Cavender-Bares, J., Potts, M., Zacharias, E. and Bazzaz, F.A. 2000. Consequences of CO2 and light interactions for leaf phenology, growth, and senescence in Quercus rubra. Global Change Biology 6: 877-887.
Cowling, S.A. and Sykes, M.T. 1999. Physiological significance of low atmospheric CO2 for plant-climate interactions. Quaternary Research 52: 237-242.
Darbah, J.N.T., Sharkey, T.D., Calfapietra, C. and Karnosky, D.F. 2010. Differential response of aspen and birch trees to heat stress under elevated carbon dioxide. Environmental Pollution 158: 1008-1014.
Eamus, D., Duff, G.A. and Berryman, C.A. 1995. Photosynthetic responses to temperature, light flux-density, CO2 concentration and vapor pressure deficit in Eucalyptus tetrodonia grown under CO2 enrichment. Environmental Pollution 90: 41-49.
Ellsworth, D.S. 1999. CO2 enrichment in a maturing pine forest: are CO2 exchange and water status in the canopy affected? Plant, Cell and Environment 22: 461-472.
Frenck, G., van der Linden, L., Mikkelsen, T.N., Brix, H. and Jorgensen, R.B. 2011. Increased CO2 does not compensate for negative effects on yield caused by higher temperature and O3 in Brassica napus L. European Journal of Agronomy 35: 127-134.
Gaucharel, C., Guiot, J. and Misson, L. 2008. Changes of the potential distribution area of French Mediterranean forests under global warming. Biogeosciences 5: 1493-1503.
Ghannoum, O., Phillips, N.G., Conroy, J.P., Smith, R.A., Attard, R.D., Woodfield, R., Logan, B.A., Lewis, J.D. and Tissue, D.T. 2010a. Exposure to preindustrial, current and future atmospheric CO2 and temperature differentially affects growth and photosynthesis in Eucalyptus. Global Change Biology 16: 303-319.
Ghannoum, O., Phillips, N.G., Sears, M.A., Logan, B.A., Lewis, J.D., Conroy, J.P. and Tissue, D.T. 2010b. Photosynthetic responses of two eucalypts to industrial-age changes in atmospheric [CO2] and temperature. Plant, Cell and Environment 33: 1671-1681.
Hamerlynck, E.P., Huxman, T.E., Loik, M.E. and Smith, S.D. 2000. Effects of extreme high temperature, drought and elevated CO2 on photosynthesis of the Mojave Desert evergreen shrub, Larrea tridentata. Plant Ecology 148: 183-193.
Hickler, T., Smith, B., Prentice, I.C., Mjofors, K., Miller, P., Arneth, A. and Sykes, M.T. 2008. CO2 fertilization in temperate FACE experiments not representative of boreal and tropical forests. Global Change Biology 14: 1531-1542.
Hickler, T., Smith, B., Sykes, M.T., Davis, M., Sugita, S. and Walker, K. 2004. Using a generalized vegetation model to simulate vegetation dynamics in northeastern USA. Ecology 85: 519-530.
Huang, J.-G., Bergeron, Y., Denneler, B., Berninger, F. and Tardif, J. 2007. Response of forest trees to increased atmospheric CO2. Critical Reviews in Plant Sciences 26: 265-283.
Huang, J.G. and Zhang, Q.B. 2007. Tree-rings and climate for the last 680 years in Wulan area of northeastern Qinghai-Tibetan Plateau. Climatic Change 80: 369-377.
Huber, M. 2008. A hotter greenhouse? Science 321: 353-354.
Huber, M. 2009. Snakes tell a torrid tale. Nature 457: 669-670.
Hymus, G.J., Ellsworth, D.S., Baker, N.R. and Long, S.P. 1999. Does free-air carbon dioxide enrichment affect photochemical energy use by evergreen trees in different seasons? A chlorophyll fluorescence study of mature loblolly pine. Plant Physiology 120: 1183-1191.
Hymus, G.J., Johnson, D.P., Dore, S., Anderson, H.P., Hinkle, C.R. and Drake, B.G. 2003. Effects of elevated atmospheric CO2 on net ecosystem CO2 exchange of a scrub-oak ecosystem. Global Change Biology 9: 1802-1812.
Idso, K.E. and Idso, S.B. 1994. Plant responses to atmospheric CO2 enrichment in the face of environmental constraints: A review of the past 10 years' research. Agricultural and Forest Meteorology 69: 53-203.
Idso, S.B., Idso, K.E., Garcia, R.L., Kimball, B.A. and Hoober, J.K. 1995. Effects of atmospheric CO2 enrichment and foliar methanol application on net photosynthesis of sour orange tree (Citrus aurantium; Rutaceae) leaves. American Journal of Botany 82: 26-30.
Idso, S.B. and Kimball, B.A. 1992. Effects of atmospheric CO2 enrichment on photosynthesis, respiration and growth of sour orange trees. Plant Physiology 99: 341-343.
Jaramillo, C., Ochoa, D., Conteras, L., Pagani, M., Carvajal-Ortiz, H., Pratt, L.M., Krishnan, S., Cardona, A., Romero, M., Quiroz, L., Rodriguez, G., Rueda, M.J., de la Parra, F., Moron, S., Green, W., Bayona, G., Montes, C., Quintero, O., Ramirez, R., Mora, G., Schouten, S., Bermudez, H., Navarrete, R., Parra, F., Alvaran, M., Osorno, J., Crowley, J.L., Valencia, V. and Vervoort, J. 2010. Effects of rapid global warming at the Paleocene-Eocene boundary on neotropical vegetation. Science 330: 957-961.
Kearney, M. 2006. Habitat, environment and niche: what are we modeling? Oikos 115: 186-191.
Keenan, T., Serra, J.M., Lloret, F., Ninyerola, M. and Sabate, S. 2011. Predicting the future of forests in the Mediterranean under climate change, with niche- and process-based models: CO2 matters! Global Change Biology 17: 565-579.
Kellomaki, S. and Wang, K.-Y. 2001. Growth and resource use of birch seedlings under elevated carbon dioxide and temperature. Annals of Botany 87: 669-682.
Kennett, J.P. and Stott, L.D. 1991. Abrupt deep-sea warming, palaeoceanographic changes and benthic extinctions at the end of the Palaeocene. Nature 353: 225-229.
Kubiske, M.E. and Pregitzer, K.S. 1996. Effects of elevated CO2 and light availability on the photosynthetic response of trees of contrasting shade tolerance. Tree Physiology 16: 351-358.
Lewis, J.D., Lucash, M., Olszyk, D. and Tingey, D.T. 2001. Seasonal patterns of photosynthesis in Douglas fir seedlings during the third and fourth year of exposure to elevated CO2 and temperature. Plant, Cell and Environment 24: 539-548.
Lewis, S.L., Malhi, Y. and Phillips, O.L. 2004. Fingerprinting the impacts of global change on tropical forests. Philosophical Transactions of the Royal Society B 359: 437-462.
Liang, E.Y., Shao, X.M., Eckstein, D., Huang, L. and Liu, X.H. 2006. Topography- and species-dependent growth response of Sabina przewalskii and Picea crassifolia to climate on the northeast Tibetan Plateau. Forest Ecology and Management 236: 268-277.
Long, S.P. 1991. Modification of the response of photosynthetic productivity to rising temperature by atmospheric CO2 concentrations: Has its importance been underestimated? Plant, Cell and Environment 14: 729-739.
Maherali, H. and DeLucia, E.H. 2000. Interactive effects of elevated CO2 and temperature on water transport in ponderosa pine. American Journal of Botany 87: 243-249.
Martinez-Vilalta, J., Lopez, B.C., Adell, N., Badiella, L. and Ninyerola, M. 2008. Twentieth century increase of Scots pine radial growth in NE Spain shows strong climate interactions. Global Change Biology 14: 2868-2881.
McMurtrie, R.E., Comins, H.N., Kirschbaum, M.U.F. and Wang, Y.-P. 1992. Modifying existing forest growth models to take account of effects of elevated CO2. Australian Journal of Botany 40: 657-677.
McMurtrie, R.E. and Wang, Y.-P. 1993. Mathematical models of the photosynthetic response of tree stands to rising CO2 concentrations and temperatures. Plant, Cell and Environment 16: 1-13.
Nigh, G.D., Ying, C.C. and Qian, H. 2004. Climate and productivity of major conifer species in the interior of British Columbia, Canada. Forest Science 50: 659-671.
Norby, R.J., DeLucia, E.H., Gielen, B., Calfapietra, C., Giardina, C.P., King, S.J., Ledford, J., McCarthy, H.R., Moore, D.J.P., Ceulemans, R., De Angelis, P., Finzi, A.C., Karnosky, D.F., Kubiske, M.E., Lukac, M., Pregitzer, K.S., Scarasci-Mugnozza, G.E., Schlesinger, W.H. and Oren, R. 2005. Forest response to elevated CO2 is conserved across a broad range of productivity. Proceedings of the National Academy of Sciences 102: 18,052-18,056.
Norby, R.J. and Luo, Y. 2004. Evaluating ecosystem responses to rising atmospheric CO2 and global warming in a multi-factor world. New Phytologist 162: 281-293.
Osorio, M.L., Osorio, J., Vieira, A.C., Goncalves, S. and Romano, A. 2011. Influence of enhanced temperature on photosynthesis, photooxidative damage, and antioxidant strategies in Ceratonia siliqua L. seedlings subjected to water deficit and rewatering. Photosynthetica 49: 3-12.
Pagani, M., Caldeira, K, Archer, D. and Zachos, J.C. 2006. An ancient carbon mystery. Science 314: 1556-1557.
Peltola, H., Kilpelainen, A. and Kellomaki, S. 2002. Diameter growth of Scots pine (Pinus sylvestris) trees grown at elevated temperature and carbon dioxide concentration under boreal conditions. Tree Physiology 22: 963-972.
Roden, J.S., Egerton, J.J.G. and Ball, M.C. 1999. Effect of elevated [CO2] on photosynthesis and growth of snow gum (Eucalyptus pauciflora) seedlings during winter and spring. Australian Journal of Plant Physiology 26: 37-46.
Royer, D.L. 2010. Fossil soils constrain ancient climate sensitivity. Proceedings of the National Academy of Sciences, USA 107: 517-518.
Sage, R.F. and Kubien, D.S. 2007. The temperature response of C3 and C4 photosynthesis. Plant, Cell and Environment 30: 1086-1106.
Sallas, L., Luomala, E.-M., Utriainen, J., Kainulainen, P. and Holopainen, J.K. 2003. Contrasting effects of elevated carbon dioxide concentration and temperature on Rubisco activity, chlorophyll fluorescence, needle ultrastructure and secondary metabolites in conifer seedlings. Tree Physiology 23: 97-108.
Shao, X.M., Huang, L., Liu, H.B., Liang, E.Y., Fang, X.Q. and Wang, L.L. 2005. Reconstructions of precipitation variation from tree-rings in recent 1000 years in Delingha, Qinghai. Science in China (Series D) 48: 939-949.
Sheu, B.-H. and Lin, C.-K. 1999. Photosynthetic response of seedlings of the sub-tropical tree Schima superba with exposure to elevated carbon dioxide and temperature. Environmental and Experimental Botany 41: 57-65.
Smith, B., Prentice, I.C. and Sykes, M.T. 2001. Representation of vegetation dynamics in the modeling of terrestrial ecosystems: comparing two contrasting approaches within European climate space. Global Ecology & Biogeography 10: 621-637.
Stoskopf, N. 1981. Understanding Crop Production. Prentice-Hall, Upper Saddle River, New Jersey, USA.
Stuhlfauth, T. and Fock, H.P. 1990. Effect of whole season CO2 enrichment on the cultivation of a medicinal plant, Digitalis lanata. Journal of Agronomy and Crop Science 164: 168-173.
Teskey, R.O. 1997. Combined effects of elevated CO2 and air temperature on carbon assimilation of Pinus taeda trees. Plant, Cell and Environment 20: 373-380.
Tewksbury, J.J., Huey, R.B. and Deutsch, C.A. 2008. Putting the heat on tropical animals. Science 320: 1296-1297.
Tissue, D.T., Thomas, R.B. and Strain, B.R. 1997. Atmospheric CO2 enrichment increases growth and photosynthesis of Pinus taeda: a 4-year experiment in the field. Plant, Cell and Environment 20: 1123-1134.
Tjoelker, M.G., Oleksyn, J. and Reich, P.B. 1998a. Seedlings of five boreal tree species differ in acclimation of net photosynthesis to elevated CO2 and temperature. Tree Physiology 18: 715-726.
Tjoelker, M.G., Oleksyn, J. and Reich, P.B. 1998b. Temperature and ontogeny mediate growth response to elevated CO2 in seedlings of five boreal tree species. New Phytologist 140: 197-210.
Turnbull, M.H., Murthy, R. and Griffin, K.L. 2002. The relative impacts of daytime and night-time warming on photosynthetic capacity in Populus deltoides. Plant, Cell and Environment 25: 1729-1737.
Turnbull, M.H., Tissue, D.T., Murthy, R., Wang, X., Sparrow, A.D. and Griffin, K.L. 2004. Nocturnal warming increases photosynthesis at elevated CO2 partial pressure in Populus deltoides. New Phytologist 161: 819-826.
Usami, T., Lee, J. and Oikawa, T. 2001. Interactive effects of increased temperature and CO2 on the growth of Quercus myrsinaefolia saplings. Plant, Cell and Environment 24: 1007-1019.
van der Meer, P.J., Jorritsma, I.T.M. and Kramer, J.K. 2002. Assessing climate change effects on long-term forest development: adjusting growth, phenology and seed production in a gap model. Forest Ecology and Management 162: 39-52.
Veteli, T.O., Mattson, W.J., Niemela, P., Julkunen-Tiitto, R., Kellomaki, S., Kuokkanen, K. and Lavola, A. 2007. Do elevated temperature and CO2 generally have counteracting effects on phenolic phytochemistry of boreal trees? Journal of Chemical Ecology 33: 287-296.
Way, D.A. and Oren, R. 2010. Differential responses to changes in growth temperature between trees from different functional groups and biomes: a review and synthesis of data. Tree Physiology 30: 669-688.
Wayne, P.M., Reekie, E.G. and Bazzaz, F.A. 1998. Elevated CO2 ameliorates birch response to high temperature and frost stress: implications for modeling climate-induced geographic range shifts. Oecologia 114: 335-342.
Wertin, T.M., McGuire, M.A. and Teskey, R.O. 2010. The influence of elevated temperature, elevated atmospheric CO2 concentration and water stress on net photosynthesis of loblolly pine (Pinus taeda L.) at northern, central and southern sites in its native range. Global Change Biology 16: 2089-2103.
Wertin, T.M., McGuire, M.A., van Iersel, M., Ruter, J.M. and Teskey, R.O. 2012. Effects of elevated temperature and [CO2] on photosynthesis, leaf respiration, and biomass accumulation of Pinus taeda seedlings at a cool and a warm site within the species' current range. Canadian Journal of Forest Research 42: 943-957.
Westerhold, T., Rohl, U., McCarren, H.K. and Zachos, J.C. 2009. Latest on the absolute age of the Paleocene-Eocene Thermal Maximum (PETM): New insights from exact stratigraphic position of key ash layers + 19 and - 17. Earth and Planetary Science Letters 287: 412-419.
Zachos, J.C., Wara, M.W., Bohaty, S., Delaney, M.L., Petrizzo, M.R., Brill, A., Bralower, T.J. and Premoli-Silva, I. 2003. A transient rise in tropical sea surface temperature during the Paleocene-Eocene Thermal Maximum. Science 302: 1551-1554.
Zeebe, R.E., Zachos, J.C. and Dickens, G.R. 2009. Carbon dioxide forcing alone insufficient to explain Palaeocene-Eocene Thermal Maximum warming. Nature Geoscience 2: 576-580.
Zhang, Q.B., Cheng, G.D., Yao, T.D., Kang, X.C. and Huang, J.G. 2003. A 2,326-year tree-ring record of climate variability on the northeastern Qinghai-Tibetan Plateau. Geophysical Research Letters 30: 10.1029/2003GL017425.
Zhang, Q.B. and Qiu, H.Y. 2007. A millennium-long tree-ring chronology of Sabina przewalskii on northeastern Qinghai-Tibetan Plateau. Dendrochronologia 24: 91-95.
Last updated 5 June 2013



