It has been claimed that CO2-induced global warming will be so great and so rapid that many of Earth's trees will not be able to migrate towards cooler regions (poleward in latitude or upward in altitude) rapidly enough to avoid extinction (Woodwell, 1989; Overpeck et al., 1991; Dobson, 1992; Root and Schneider, 1993; Dyer, 1995). This prediction is based on the assumption that tree growth rates rise from zero at the cold limit of their natural ranges (their northern boundaries in the Northern Hemisphere) to a broad maximum, after which they decline to zero at the warm limits of their natural ranges (their southern boundaries in the Northern Hemisphere). Loehle (1998), however, convincingly demonstrated that this assumption is only half correct: it properly describes tree growth dynamics near a Northern Hemispheric forest's northern boundary; but it is an inaccurate representation of tree growth dynamics near such a forest's southern boundary.
In his treatise on the subject, for example, Loehle noted that in the Northern Hemisphere trees planted north of their natural ranges' northern boundaries are only able to grow to maturity within 50-100 miles of those boundaries. Trees planted south of their natural ranges' southern boundaries, however, often grow to maturity as much as 1000 miles further south (Dressler, 1954; Woodward, 1987, 1988). In fact, Loehle reported that "many alpine and arctic plants are extremely tolerant of high temperatures; and in general one cannot distinguish between arctic, temperate, and tropical-moist-habitat types on the basis of heat tolerances, with all three types showing damage at 44-52°C (Gauslaa, 1984; Lange and Lange, 1963; Levitt, 1980; Kappen, 1981)."
What Loehle found from his review of the literature, and from his own experience with U.S. trees, was that as temperatures and growing degree days rise from very low levels, boreal tree growth rates at some point begin to rise from zero and continue increasing until they either plateau out at some maximum value or drop only very slowly thereafter as temperatures rise still higher and growing degree days continue to accumulate. Trees from the U.S. Midwest, by comparison, do not begin to grow until a higher temperature or greater accumulation of growing degree days is reached; but their growth rates rise much higher than those of the colder-adapted boreal species before they too either level out or begin to decline ever so slowly. Last of all, southern species do not begin to grow until even higher temperatures or growing degree day sums are reached, after which their growth rates rise highest of all before leveling out and exhibiting essentially no decline thereafter.
In light of these observations, it can readily be appreciated that although the northern range limit of a particular tree species is indeed determined by growth-retarding cool growing seasons and frost damage, the southern boundary of a tree's natural range is not determined by temperature per se, but by competition between the northern species and more southerly-adapted species that have inherently greater growth rates.
Whenever significant long-term warming occurs, therefore, Earth's coldest-adapted trees are presented with an opportunity to rapidly extend their ranges northward in the Northern Hemisphere. Individual trees at the southern limits of their ranges, however, feel no need to go anywhere. As time progresses, however, they may at some point begin to experience pressure from some of the "stronger" nearest-neighbor southern species trying to invade their territory; but this potential challenge is by no means assured of quick success; for as Loehle describes it:
- Seedlings of these southern species will not gain much competitive advantage from faster growth in the face of existing stands of northern species, because the existing adult trees have such an advantage due to light interception. Southern types must wait for gap replacement, disturbances, or stand break up to utilize their faster growth to gain a position in the stand. Therefore, the replacement of species will be delayed at least until the existing trees die, which can be hundreds of years. Furthermore, the faster growing southern species will be initially rare and must spread, perhaps across considerable distances or from initially scattered localities. Thus, the replacement of forest (southern types replacing northern types) will be an inherently slow process (several to many hundreds of years).
In summing up the significance of this situation, Loehle stated that "forests will not suffer catastrophic dieback due to increased temperatures but will rather be replaced gradually by faster growing types."
Another possibility is that northern or high-altitude forests will not be replaced at all by southern or low-altitude forests. Rather, they may merge, creating new types of forests of greater species richness, such as those of the warmer Tertiary, when in the western U.S. many montane taxa regularly grew among mixed conifers and broadleaf schlerophylls (Axelrod 1944a, 1944b, 1956, 1987), creating what could well be called super forest ecosystems, which Axelrod (1988) aptly described as "much richer than any that exist today." Possibly helping warmer temperatures to produce this phenomenon during the Tertiary were the higher atmospheric CO2 concentrations of that period (Volk, 1987), as suggested by Idso (1989); and that condition is something that is also expected to occur in the future.
These many real-world observations make it abundantly clear that any global warming that might occur in the future will not create an irresistible impetus for the warm-limit boundaries of forests to move poleward or upward. In addition, a further increase in the air's CO2 content, which is almost assured, would prevent such a phenomenon from occurring anyway; for as noted in the title of the paper of Taub et al. (2000), "growth in elevated CO2 protects photosynthesis against high-temperature damage." In fact, not only does elevated CO2 protect plants at high temperatures, it actually makes them prefer warmer environments; for as Idso and Idso (1994) demonstrated in their review of the literature, and as has been discussed by Long (1991) and Cannell and Thornley (1998), the optimum temperature for plant growth typically rises, and by several degrees C, in response to a 300-ppm increase in the air's CO2 concentration, as does the temperature at which heat-induced death is induced in plants (Idso et al., 1995). And so it is that climate alarmists have got things 180 degrees out of phase with reality. Higher temperatures, especially if accompanied by higher atmospheric CO2 concentrations, will not only not reduce the species richness of Earth's forests, they will likely enhance it.
Transiting into the 21st century, Bergh et al. (2003) used a boreal version of a process-based simulation model (BIOMASS) to quantify the individual and combined effects of elevated air temperature (2 and 4°C above ambient) and CO2 concentration (350 ppm above ambient) on the net primary production (NPP) of both coniferous (Pinus sylvestris, Picea abies) and deciduous broad-leaf (Fagus sylvatica, Populus trichocarpa) forests growing in Denmark, Finland, Iceland, Norway and Sweden. For three of the four species (P. sylvestris, P. abies, P. trichocarpa), air temperature increases of 2 and 4°C led to mean NPP increases of 11 and 20%, respectively; while for the other species (F. sylvatica) there were 21 and 48% decreases in NPP. However, when the air's CO2 concentration was simultaneously increased from 350 to 700 ppm, the corresponding mean NPP increases of the three species rose to 41 and 55%; while the NPP of F. sylvatica jumped from -21 and -48% to +37 and +10%. Last of all, when the air's CO2 content was doubled at the prevailing ambient temperature, the mean value of the NPP of the three species rose by 27%, while that of F. sylvatica rose by 58%. Thus, as the air's CO2 content continues to climb, the major tree species of Denmark, Finland, Iceland, Norway and Sweden should grow ever more productively; and if air temperature also rises, most of them will grow better still.
Perfors et al. (2003) used a set of overhead infrared radiative heaters to continuously warming five 3- x 10-meter plots of ungrazed montane meadow at the Rocky Mountain Biological Laboratory in Gunnison County, Colorado, USA, while five similar plots have served as controls. The extra downward flux of infrared radiation warmed the top 15 cm of soil by about 1.5°C and dried it by about 15% on a gravimetric basis during the growing season, prolonging the snow-free season at each end by a total of about 20 days. Under these conditions the authors extracted the age-detrended growth rate of common sagebrush -- Artemisia tridentata (Nutt.), ssp. vaseyana -- a perennial shrub that is abundant throughout much of the semiarid western United States, in an effort to determine the effect of a modest warming on the distribution of this common woody plant. In doing so they report that annual sagebrush growth rates in the heated plots were approximately 50% greater than those in the control plots, due primarily to the warming-induced increase in the length of the snow-free season. Perfors et al. say their observations and analysis "suggest that global climate change, which is expected to result in a contracted period of snow accumulation in the montane west, will result in increased growth and range expansion of sagebrush near high-elevation range boundaries in the western US." Additionally, although it had earlier been demonstrated that the experimental warming treatment decreased soil organic carbon content (Saleska et al., 2002), Perfors et al. further suggest that, over the long haul, "because sagebrush litter is more recalcitrant to decomposition than is the litter from the forb species that are in decline in the heated plots of our climate manipulation experiment, enhanced sagebrush growth could also contribute to a negative feedback [to CO2-induced warming] by increasing the turnover time of soil carbon."
Shortly thereafter, Esper and Schweingruber (2004) introduced their study of real-world tree responses to temperature changes by noting that "tree recruitment is related to some combination of temperature variations, micro-site conditions, insect outbreaks, winter-time snow and wind conditions and grazing pressure," citing Payette (1974), Payette and Filion (1985), Stocklin and Korner (1999) and Holtmeier (2000), but also reporting that growing season temperature alone is a major determinant of global treeline limits, citing Brockmann-Jerosch (1919) and Korner (1998). And on this latter basis they went on to analyze treeline dynamics over western Siberia during the 20th century by comparing nine undisturbed polar sites located between 59 and 106°E and 61 and 72°N and merging information from those sites in such a way that, in their words, "larger-scale patterns of treeline changes are demonstrated, and related to decadal-scale temperature variations." In addition, they related current treeline positions to former treeline locations "by documenting in-situ remnants of relict stumps and logs."
This work revealed two main pulses of northward treeline advance during the 20th century. The first occurred between 1940 and 1960, while the second started around 1972 and lasted into the 1980s. These treeline advances corresponded closely to decadal-scale annual temperature increases; and Esper and Schweingruber remarked that the lack of germination events prior to the mid-20th century indicated that these were "exceptional advances." Nevertheless, they indicated that the relict stumps and logs found at most sites showed that these advances were part of a long-term reforestation of tundra environments, noting that "stumps and logs of Larix sibirica can be preserved for hundreds of years (Shiyatov, 1992)," and that "above the treeline in the Polar Urals such relict material from large, upright trees were sampled and dated, confirming the existence, around AD 1000, of a forest treeline 30 m above the late 20th century limit (Shiyatov, 2003)." And they also noted that "this previous forest limit receded around 1350, perhaps caused by a general cooling trend (Briffa, 2000; Esper et al., 2002."
"Synchronous with the advance shown from the western Siberian network," according to the two researchers, a mid-20th century tree recruitment period was also occurring in "central Sweden (Kullmann, 1981), northern Finland (Kallio, 1975), northern Quebec (Morin and Payette, 1984) and the Polar Urals (Shiyatov, 1992)." And together with their own results from Asia, they thus concluded that "these findings from Europe and North America support a circumpolar trend, likely related to a global climate warming pattern," thereby demonstrating the positive response of the biosphere to the warming that accompanied the demise of the Little Ice Age and the establishment of the Current Warm Period. In addition, they demonstrated the existence of the warmer-than-present multi-century period centered around AD 1000 that we know today as the Medieval Warm Period.
And what was found to be occurring throughout the trees of Nordic regions was also found to be occurring among the trees of Earth's tropics. In a major review of the subject, for example, Malhi and Phillips (2004) reported that Phillips et al. (2004) had confirmed that "turnover rates appear to have accelerated across Amazonia," and that Lewis et al. (2004) - who examined simultaneous changes in forest biomass, growth, mortality and stem number in 50 Amazonian forest plots - demonstrated that "there appears to have been an acceleration of growth in most of these plots, accompanied by a lagged acceleration of mortality and a general increase in biomass and stem number." And in commenting on these findings, Malhi and Phillips said they implied "a direct forcing from CO2, solar radiation and/or possibly temperature."
Back in Earth's boreal regions, Chen et al. (2006) analyzed 13 years (1990-1996, 1999-2004) of hourly-averaged atmospheric CO2 concentration data obtained from a 40-m tower at Fraserdale, Ontario, Canada (together with temperature, humidity and wind speed measured at 20 and 40 meters and precipitation at ground level), comparing their results with a marine boundary layer CO2 data set representing the free troposphere above the tower. This effort revealed that in warmer years, the planetary boundary layer over their measurement site was more depleted of CO2, which suggests that the 103-104 km2 land area of the boreal ecosystem upwind of the tower sequestered more carbon in such years; and they said that this finding "suggests that gross primary productivity increased considerably faster with temperature than did ecosystem respiration," which relationship they found to be true for both annual temperatures (from year to year) and 10-day mean temperatures (throughout the growing season). And, therefore, in the words of the scientists who conducted the research, "the fact that the temperature sensitivity of gross primary productivity is larger than that of ecosystem respiration suggests that global warming could lead to increased carbon sequestration in boreal ecosystems."
In another boreal region study, this one conducted in the Komi Republic of northwestern Russia, where large areas of natural boreal forest still exist, Lopatin (2007) analyzed the apical growth history of 108 Scots pine (Pinus sylvestris L.) and 88 Siberian spruce (Picea obovata Ledeb.) trees via cohort comparison, whereby "differences in average height growth curves between trees with different germination dates were used as indicators [of] changes in forest site productivity over time." Focusing on two age classes - 1900-1949 and 1950-2000 (based on year of germination) - this work revealed, in Lopatin's words, that "statistically significant height increment increases were found in the middle taiga zone for Siberian spruce of 240% and Scots pine of 140%, while northern taiga Siberian spruce increased by 164%. And for the entire Komi Republic (the forested area of which comprises 33% of northwest Russia's total forest area), Lopatin further stated that "a statistically significant increase in height increment of 40% for Siberian spruce and 30% for Scots pine was identified."
Taking account of the fact that the sampled trees were located in remote untouched pristine forests, Lopatin wrote that "the main causes of increased height increment are suggested to be climatic," as there was an increase in the region's temperature of 0.43°C during the prior 30 years. In addition, the atmosphere's CO2 concentration rose by close to 50 ppm over the same time period. Hence, it would appear that the twin evils of the radical environmentalist movement (rising air temperatures and CO2 concentrations) have actually been a blessing for the boreal forests of the Komi Republic.
In a model based study, Davi et al. (2006) introduce their work by noting that "predictions for the second half of the 21st century diverge, with some models predicting that the terrestrial carbon sink will tend to level off, while others predict a decrease [our italics]," noting, in this regard, that "forest ecosystems play a dominant role in controlling terrestrial carbon sinks." Hoping to shed more light on this important subject, Davi et al. used a meteorological model following "a moderate CO2 emission scenario" - B2 of the IPCC - to calculate a 1960-2100 average temperature increase of 3.1°C and a mean summer rainfall decrease of 27%, which they used as input to a physiologically-based multi-layer process-based ecosystem productivity model (which contained a carbon allocation sub-model coupled with a soil model) to evaluate net productivity changes of six French forest ecosystems representative of oceanic, continental and Mediterranean climates that are dominated, respectively, by deciduous species (Fagus sylvatica, Quercus robur), coniferous species (Pinus pinaster, Pinus sylvestris) and sclerophyllous evergreen species (Quercus ilex), which ecosystems, in their words, "are representative of a significant proportion of forests in western Europe."
"By comparing runs with and without CO2 effects," according to the researchers, they found that "CO2 fertilization is responsible from 1960 to 2100 for an NEP [net ecosystem productivity] enhancement of about 427 g(C) on average for all sites (= 3.05 g(C) m-2 year-1)," noting that "the CO2 fertilization effect" actually turns a warming- and drying-induced "decrease of NEP into an increase." In addition, they report that "no saturation of this effect on NEP is found because the differences between the simulations with and without CO2 fertilization continuously increase with time." Thus, even in the face of what truly would be an "unprecedented" global warming and drying scenario, the real-world physiological effects of atmospheric CO2 enrichment that are included in the ecosystem productivity model employed by Davi et al. are able to more than compensate for the deleterious effects of the dramatic climate-change scenario on the productivity of major European forests.
Jumping ahead three years, we come to the illuminating study of Gunderson et al. (2010), who in discussing the long-term impacts of atmospheric warming on forest productivity and composition, wrote that "because range boundaries often follow temperature gradients, it is inferred that species differ in temperature sensitivity, such that climatic warming would cause extensive range shifts and local extinctions." But is this really so?
Noting that "without data to suggest otherwise," the five researchers reported that then-current forest biogeography simulations typically used "conditions in a species' current range (its climate envelope) to suggest limits for its growth and survival." This concept, however, had been severely critiqued in many quarters; and, hence, the researchers from the Environmental Sciences Division of the Oak Ridge National Laboratory in Tennessee (USA) set about to see if they too might possibly obtain "data to suggest otherwise."
In their approach to the subject, Gunderson et al. "investigated photosynthetic sensitivity to temperature and the potential for acclimation in relation to the climatic provenance of five species of deciduous trees, Liquidambar styraciflua [sweetgum], Quercus rubra [northern red oak], Quercus falcata [southern red oak], Betula alleghaniensis [yellow birch] and Populus grandidentata [bigtooth aspen]." This they did out-of-doors within open-top chambers that were maintained at three different temperature regimes -- ambient and ambient plus 2 and 4°C above ambient -- for a period of three years, "tracking natural temperature variability," as they described it, which occurred throughout days, over seasons and among years.
When all was said and done, the five scientists reported that "warming treatments resulted in a shift in the temperature response curves for CO2 assimilation, such that tree leaves in warmer treatments had higher temperature optima [Topt]," an example of which phenomenon is depicted in the figure below for Q. rubra seedlings during one specific month.
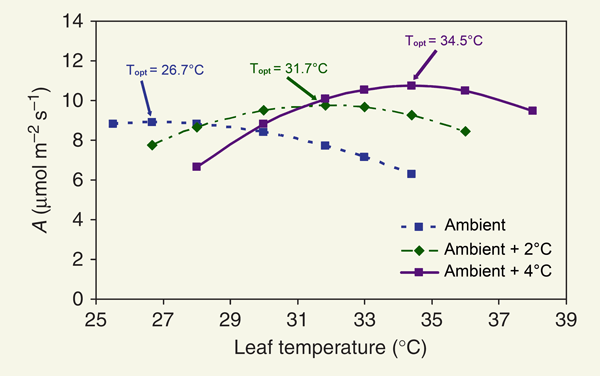
Figure 1. Net CO2 assimilation rate (A) vs. leaf temperature in Quercus rubra seedlings during June of 2003 in the ambient temperature (TA) and elevated temperature (TA +2°C and TA +4°C) treatments. Adapted from Gunderson et al. (2010).
As may be seen from this figure, the trees growing in progressively warmer environments had progressively higher Topt values; and there was a tendency for the net CO2 assimilation rates at those higher Topt values to be a bit higher as well. Gunderson et al. additionally noted that this adjustment in photosynthetic response was typically accomplished over a period of as little as two days; while indicating that "others have found all or most acclimation within 2-6 days (Veres and Williams, 1984; Hill et al., 1988; Battaglia et al., 1996; Turnbull et al., 2002; Froux et al., 2004)." They also stated that a similar "adjustment of thermal optima was confirmed in all species, whether temperatures varied with season or treatment, and regardless of climate in the species' range or provenance of the plant material," and they said that the observed "responses to the temperature manipulation were not different from the seasonal acclimation observed in mature indigenous trees," which they also investigated locally.
"In all species tested," in the words of Gunderson et al., photosynthetic optima "shifted with air temperature, improving carbon gain during otherwise non-optimal portions of the growing season, and moderating the impact of a 2-4°C increase in temperature," and they remarked that "the strong acclimation potential observed in this investigation suggests that seedling physiology is not as sensitive to warming per se as predicted by original algorithms." Thus, they suggested that "photosynthetic responses should not be modeled using static temperature functions," or what many call climate envelopes, "but should incorporate an adjustment to account for acclimation," noting that "the high degree of homeostasis observed indicates that direct impacts of climatic warming on forest productivity, species survival, and range limits may be less than predicted by existing models."
In light of these several observations, it would indeed appear that Earth's trees should be able to successfully adjust their physiology to accommodate a warming of the magnitude and rate-of-rise that is typically predicted by climate alarmists to accompany the projected future increase in the air's CO2 content. In fact, if the planet's trees can adjust their physiology to not only survive an instantaneous warming of 2-4°C, but to actually benefit from it, as has been demonstrated to be the case by Gunderson et al., they would probably relish the opportunity to respond to a similar warming projected to develop over the course of a century or more. And when one factors in the aerial fertilization effect of the rising atmospheric CO2 concentration, plus its transpiration-reducing effect that boosts tree water use efficiency, as well as CO2's ability to also raise the Topt values of most plants, the world's forests are seen to possess the ideal mix of multiple ingredients to support a tremendous "greening of the Earth."
A short time later, Wang et al. (2012) wrote that "competition with neighboring plants is one of the most important biotic factors limiting plant growth," noting that "plants in a stand compete with each other for resources" and that "competition alters morphological and physiological traits of plants," citing Hikosaka et al. (1999, 2003, 2005) and Nagashima et al. (2003). And they stated that these alterations, in turn, "result in changes in the microclimate and resource acquisition in the stand, and consequently influence the growth and development of individuals," which led them to conclude that "it is indispensable to study temperature responses of plants growing in a stand instead of growing individually."
Heeding their own advice - and within each of six enclosed-top fumigation chambers erected out-of-doors at the Maoxian Ecological Station in southwest China - Wang et al. placed a single wooden box filled with surface sandy soil taken from a 30-year-old Aibes faxoniana forest; and they planted within each of them same-size 7-year-old A. faxoniana seedlings at a density of 16 plants per square meter, while draping high-density shade cloths over the tops of the chambers so that the light intensity received by the seedlings was about 35% of ambient top-of-the-forest light intensity, in order to mimic the understory light environment in which A. faxoniana seedlings typically grow. And for the following six years, they monitored numerous plant properties, while half of the chambers were maintained at the ambient outdoor temperature (T) and the other half were maintained at T + 2.2°C.
At the end of their study, the three Chinese scientists were able to report that "warming caused statistically significant increases in the specific leaf area, leaf area ratio, root biomass, leaf biomass, branch biomass, stem biomass, and total mass of the seedlings," as well as "total chlorophyll concentrations, specific chlorophyll pigments, and chlorophyll a/b ratios," and they reported that these changes in branch growth and needle chemistry enhanced the light-capture potential of seedlings growing in the low-light environment typical of Faxon fir understories. And as a result, the three researchers concluded that "future elevated temperature may adjust trees' morphology and physiology to enable the capture of more light to support seedling growth under growth-limiting light, intra-specific competition and nutritional conditions."
Also publishing about this same time were Lugo et al. (2012), who wrote that with late-20th-century changes in temperature, "variations in phenology have acquired particular importance," and they noted, in this regard, that the longest monitoring periods of plants have been carried out in the botanical gardens of temperate Europe and deal with the effects of temperature changes on the growth dynamics of primary meristems (buds, leaves and flowers), while there has been no historical documentation relative to the phenology of the secondary meristem or cambium, as it is not a macroscopically-perceptible phenomenon like leaf development or flower maturation. So in an attempt to begin filling this research void, Lugo et al. monitored the timing of xylogenesis (wood formation) in black spruce (Picea mariana) trees for nine years on a weekly basis at four sites in the boreal forest of Quebec, Canada. This they did in order to reconstruct the onset, duration and ending of xylogenesis in trees of this species between 1950 and 2010, as well as the relationships of these phenomena to chronologies of maximum and minimum air temperatures.
The three researchers first reported that "all sites exhibited increasing trends of both annual and May-September temperatures, with the greatest changes observed at the higher latitudes," after which they stated that "phenological events in spring were more affected than those occurring in autumn, with cambial [growth] resumptions occurring 0.5-0.8 days/decade earlier." And they added that if the observed trend is maintained unaltered in the long term, "the demonstrated advancement of cambial activity could dramatically modify the short time window for growth of boreal species and markedly affect cell production of the secondary meristem," with the result that "long-term increases in temperature can substantially extend wood formation and, consequently, the dynamics and productivity of cold ecosystems, by removing the thermal constraints to the activity of carbon sinks in trees."
Introducing their work, Krause et al. (2013) write that "information on thermal tolerance and the ability of plant species in the humid tropics to acclimate to altered high temperature regimes is scarce and largely restricted to a small number of important agronomic species." And, therefore, they decided to add to this "small number" of studied species, while simultaneously shifting their attention to a neotropical pioneer tree species, Ficus insipida. To do so, they collected seeds of F. insipida from mature trees and germinated them in January 2012 in peat pellets until they developed 4 or 5 leaves, after which they were transferred into 2.2-L plastic pots filled with a commercial potting mix, given an initial dose of slow release fertilizer and thereafter watered to field capacity daily. This was done while the plants were maintained in controlled-environment chambers, where air temperature was held steady at 39°C during each 12-hour light period and at either 32 or 22°C during each 12-hour dark period. And at various times during their 39-day study, Krause et al. measured certain plant physiological parameters and processes, after which total plant biomass was determined.
According to the five researchers, their results showed that "seedlings cultivated at 39/32°C exhibited much faster growth than seedlings grown at 39/22°C." In fact, they say "total biomass accumulation was about three times higher in plants grown at the elevated nighttime temperature, as compared to biomass accumulation at 39/22°C," while their actual data indicate total biomass accumulation was 3.25 times faster. In discussing their findings, Krause et al. write that their most striking result was "the profound increase in biomass accumulation of plants under elevated nighttime temperature," which "contrasts with the widely assumed negative impact of increased nighttime temperature upon tropical tree growth and the general paradigm that warmer temperatures reduce tree growth in the tropics." Consistent with their findings, however, they note that "nighttime warming has been shown to stimulate growth in tobacco (Camus and Went, 1952), cotton (Koniger and Winter, 1993), and in another tropical pioneer tree species, Ochroma pyramidale (A. Cheesman and K. Winter, unpublished data)." They also note that "increased night-time temperatures have been implicated in increased growth of red oak seedlings in urban environments in upstate New York (Searle et al., 2012)." And if these results ultimately turn out to be representative of the majority of plants, it will bode well for Earth's plant life, in that the warming of the last few decades of the 20th century typically has found minimum nighttime temperatures rising faster than maximum daytime temperatures.
Also investigating the effects of nighttime warming on trees was Cheesman and Winter (2013a), who write that "contemporary global warming has been accompanied by the narrowing of the diel (24 hour) temperature range by the asymmetric rise in night-time and daytime temperatures (Kukla and Karl, 1993; Easterling et al., 1997)," and they say that "it is likely that the emergence of novel temperature regimes in the tropics (Diffenbaugh and Scherer, 2011) will include the continued asymmetric rise of night-time temperatures (IPCC, 2007), with potentially profound implications upon plant growth in an already compromised ecosystem (Wright, 2010)."
Against this backdrop, Cheesman and Winter collected seeds of two fast-growing tropical pioneer tree species - Ficus insipida and Ochroma pyramidale - from forests surrounding Panama City, and grew the seedlings the seeds produced in controlled-environment chambers at a constant daytime temperature (33°C) and at a range of increasing night-time temperatures (22, 25, 28 and 31°C) for 38 days in the case of O. pyramidale and for 54 days in the case of F. insipida, after which they harvested the young trees and determined their total biomass production. They report that in going from the coldest to the warmest night-time temperature treatment, total tree biomass accumulation rose from a mean of 0.85 g per seedling to a mean of 2.23 g per seedling in F. insipida; while in O. pyramidale, corresponding biomass accumulation values were 1.21 g and 2.65 g. And these enhancements in growth rates in response to rising night-time temperatures even occurred in spite of warming-induced increases in leaf-level dark respiration rates! And in light of these findings, the two researchers state that "contrary to the notion of adverse effects of increasing night-time temperatures on tropical tree performance (Clark et al., 2003, 2010), our results demonstrate that under well-watered conditions elevated night-time temperature promotes growth in seedlings of two neotropical pioneer tree species ... even at 31°C, far in excess of night-time temperatures currently seen in lowland Panama."
Lastly, writing as background for their work, Cheesman and Winter (2013b) say "it is hypothesized that tropical trees have adapted to operate within a narrow range of temperatures with only a limited potential to acclimate," citing Janzen (1967) and Ghalambor et al. (2006). And they also note that "climate envelope or species distribution models often highlight the narrow range of temperatures to which tropical lowland forests are restricted, emphasizing their vulnerability to increasing temperatures," citing Colwell et al. (2008), Wright et al. (2009), Laurance et al. (2011) and Zelazowski et al. (2011). To further explore this subject, the two researchers conducted experiments related to the thermal niches of seedlings of ten neo-tropical tree species under favorable conditions (i.e. medium light levels, high water and nutrient availability) and a range of temperature regimes within plant growth chambers. And they followed this set of experiments with more detailed studies of the thermal acclimation - or not - of three species at temperature regimes known to be sub- and super-optimal for growth. And what did they learn?
In their first set of experiments, the two Smithsonian Tropical Research Institute scientists found that "under well-watered conditions, all species showed optimal growth at temperatures above those currently found in their native ranges," with the exception of two species that did not survive under the highest temperature treatment. And they thus report that "clear and novel evidence" was provided that "certain tropical trees do have an ability to maintain growth as seedlings at temperatures above those of their current home range, with a fundamental thermal niche generally broader than that realized in the field," noting that "clear evidence is also provided that thermal acclimation of leaf-level processes may play an important role in this response." And they buttress the validity of these conclusions by noting that "in the small number of empirical studies that have sought to elucidate fundamental thermal niches of tropical tree species, maximum growth has often been found under the highest temperature regime tested," citing Herwitz (1993), Cunningham and Read (2003), Allen and Vu (2009), Esmail and Oelbermann (2012), and Cheesman and Winter (2013a)," adding that "in these cases, the thermal optimum for growth was found to be higher than that predicted by either climatic home range or photosynthetic thermal optimum," citing Cunningham and Read (2002). Thus the take home message of their work is that tropical trees are likely much more able to successfully deal with rising temperatures than many people have long supposed.
In conclusion, and in light of the findings of the several studies reviewed above, it would appear that Earth's trees have nothing to fear from any future global warming that might possibly occur, other than climate-alarmist-manufactured fear itself. In fact, their physiology is such that they would much more likely find that such a warming would actually enhance their rates of growth and their degrees of development.
References
Allen, L.H. and Vu, J.C.V. 2009. Carbon dioxide and high temperature effects on growth of young orange trees in a humid, subtropical environment. Agricultural and Forest Meteorology 149: 820-830.
Axelrod, D.I. 1944a. The Oakdale flora (California). Carnegie Institute of Washington Publication 553: 147-166.
Axelrod, D.I. 1944b. The Sonoma flora (California). Carnegie Institute of Washington Publication 553: 167-200.
Axelrod, D.I. 1956. Mio-Pliocene floras from west-central Nevada. University of California Publications in the Geological Sciences 33: 1-316.
Axelrod, D.I. 1987. The Late Oligocene Creede flora, Colorado. University of California Publications in the Geological Sciences 130: 1-235.
Axelrod, D.I. 1988. An interpretation of high montane conifers in western Tertiary floras. Paleobiology 14: 301-306.
Battaglia, M., Beadle, C. and Loughhead, S. 1996. Photosynthetic temperature responses of Eucalyptus globulus and Eucalyptus nitens. Tree Physiology 16: 81-89.
Bergh, J., Freeman, M., Sigurdsson, B., Kellomaki, S., Laitinen, K., Niinisto, S., Peltola, H. and Linder, S. 2003. Modeling the short-term effects of climate change on the productivity of selected tree species in Nordic countries. Forest Ecology and Management 183: 327-340.
Briffa, K.R. 2000. Annual climate variability in the Holocene: Interpreting the message of ancient trees. Quaternary Science Reviews 19: 87-105.
Brockmann-Jerosch, H. 1919. Beitrage zur Geobotanischen Landesauf-nahme, Vol. 6, Baumgrenze und Klimacharakter. Rascher, Zurich, Switzerland.
Camus, G.C. and Went, F.W. 1952. Thermoperiodicity of three varieties of Nicotiana tabacum. American Journal of Botany 38: 521-528.
Cannell, M.G.R. and Thornley, J.H.M. 1998. Temperature and CO2 responses of leaf and canopy photosynthesis: a clarification using the non-rectangular hyperbola model of photosynthesis. Annals of Botany 82: 883-892.
Cheesman, A.W. and Winter, K. 2013a. Elevated night-time temperatures increase growth in seedlings of two tropical pioneer tree species. New Phytologist 197: 1185-1192.
Cheesman, A.W. and Winter, K. 2013b. Growth response and acclimation of CO2 exchange characteristics to elevated temperatures in tropical tree seedlings. Journal of Experimental Botany 64: 3817-3828.
Chen, J.M., Chen, B., Higuchi, K., Liu, J., Chan, D., Worthy, D., Tans, P. and Black, A. 2006. Boreal ecosystems sequestered more carbon in warmer years. Geophysical Research Letters 33: 10.1029/2006GL025919.
Clark, D.B., Clark, D.A. and Oberbauer, S.F. 2010. Annual wood production in a tropical rain forest in NE Costa Rica linked to climatic variation but not to increasing CO2. Global Change Biology 16: 747-759.
Clark, D.A., Piper, S.C., Keeling, C.D. and Clark, D.B. 2003. Tropical rain forest tree growth and atmospheric carbon dynamics linked to interannual temperature variation during 1984-2000. Proceedings of the National Academy of Sciences, USA 100: 5852-5857.
Colwell, R.K., Brehm, G., Cardelus, C.L., Gilman, A.C. and Longino, J.T. 2008. Global warming, elevational range shifts, and lowland biotic attrition in the wet tropics. Science 322: 258-261.
Cunningham, S.C. and Read, J. 2002. Comparison of temperate and tropical rainforest tree species: photosynthetic responses to growth temperature. Oecologia 133: 112-119.
Cunningham, S.C. and Read, J. 2003. Comparison of temperate and tropical rainforest tree species: growth responses to temperature. Journal of Biogeography 30: 143-153.
Davi, H., Dufrene, E., Francois, C., Le Maire, G., Loustau, D., Bosc, A., Rambal, S., Granier, A. and Moors, E. 2006. Sensitivity of water and carbon fluxes to climate changes from 1960-2100 in European forest ecosystems. Agricultural and Forest Meteorology 141: 35-56.
Diffenbaugh, N.S. and Scherer, M. 2011. Observational and model evidence of global emergence of permanent, unprecedented heat in the 20th and 21st centuries. Climatic Change 197: 615-624.
Dobson, A. 1992. Withering heats: Global warming will exact a heavy toll on Earth's biodiversity. Natural History 101(9): 2-8.
Dressler, R.L. 1954. Some floristic relationships between Mexico and the United States. Rhodora 56: 81-96.
Dyer, J.M. 1995. Assessment of climatic warming using a model of forest species migration. Ecological Modelling 79: 199-219.
Easterling, D.R., Horton, B., Jones, P.D., Peterson, T.C., Karl, T.R., Parker, D.E., Salinger, M.J., Razuvayev, V., Plummer, N., Jamason, P. and Folland, C.K. 1997. Maximum and minimum temperature trends for the globe. Science 277: 364-367.
Esmail, S. and Oelbermann, M. 2011. The impact of climate change on the growth of tropical agroforestry tree seedlings. Agroforestry Systems 83: 235-244.
Esper, J., Cook, E.R. and Schweingruber, F.H. 2002. Low-frequency signals in long tree-ring chronologies for reconstructing past temperature variability. Science 295: 2250-2253.
Esper, J. and Schweingruber, F.H. 2004. Large-scale treeline changes recorded in Siberia. Geophysical Research Letters 31: 10.1029/2003GL019178.
Froux, F., Ducrey, M., Epron, D. and Dreyer, E. 2004. Seasonal variations and acclimation potential of the thermostability of photochemistry in four Mediterranean conifers. Annals of Forest Science 61: 235-236.
Gauslaa, Y. 1984. Heat resistance and energy budget in different Scandinavian plants. Holarctic Ecology 7: 1-78.
Ghalambor, C.K., Huey, R.B., Martin, P.R., Tewksbury, J.J. and Wang, G. 2006. Are mountain passes higher in the tropics? Janzen's hypothesis revisited. Integrative and Comparative Biology 46: 5-17.
Gunderson, C.A., O'Hara, K.H., Campion, C.M., Walker, A.V. and Edwards, N.T. 2010. Thermal plasticity of photosynthesis: the role of acclimation in forest responses to a warming climate. Global Change Biology 16: 2272-2286.
Herwitz, S.R. 1993. Growth rates of selected Australian tropical rain-forest tree species under controlled conditions. Oecologia 96: 232-238.
Hikosaka, K., Onoda, Y., Kinugasa, T., Nagashima, H., Anten, N.P.R. and Hirose, T. 2005. Plant responses to elevated CO2 concentration at different scales: leaf, whole plant, canopy, and population. Ecological Research 20: 243-253.
Hikosaka, K., Sudoh, S. and Hirose, T. 1999. Light acquisition and use by individuals competing in a dense stand of an annual herb, Xanthium canadense. Oecologia 118: 388-396.
Hikosaka, K., Yamano, T., Nagashima, H. and Hirose, T. 2003. Light-acquisition and use of individuals as influenced by elevated CO2 in even-aged monospecific stands of Chenopodium album. Functional Ecology 17: 786-795.
Hill, R.S., Read, J. and Busby, J.R. 1988. The temperature-dependence of photosynthesis of some Australian temperate rainforest trees and its biogeographical significance. Journal of Biogeography 15: 431-449.
Holtmeier, F.K. 2000. Arbeiten aus dem Institut fur Landschaftsokologie, Vol. 8, Die Hohengrenze der Gebirgswalder, Institut fur Landschaftsokologie, Munster, Germany.
Idso, K.E. and Idso, S.B. 1994. Plant responses to atmospheric CO2 enrichment in the face of environmental constraints: a review of the past 10 years' research. Agricultural and Forest Meteorology 69: 153-203.
Idso, S.B. 1989. Carbon Dioxide and Global Change: Earth in Transition. IBR Press, Tempe, AZ.
Idso, S.B., Idso, K.E., Garcia, R.L., Kimball, B.A. and Hoober, J.K. 1995. Effects of atmospheric CO2 enrichment and foliar methanol application on net photosynthesis of sour orange tree (Citrus aurantium; Rutaceae) leaves. American Journal of Botany 82: 26-30.
Janzen, D.H. 1967. Why mountain passes are higher in the tropics. American Naturalist 101: 233-249.
Kallio, P. 1975. Reflections on the adaptations of organisms to the northern forest limit in Fennoscandia. Paper Presented at the Circumpolar Conference on Northern Ecology, National Research Council, Ottawa, Canada.
Kappen, L. 1981. Ecological significance of resistance to high temperature. In: Lange, O.L., Nobel, P.S., Osmond, C.B. and Ziegler, H. (Eds.), Physiological Plant Ecology. I. Response to the Physical Environment. Springer-Verlag, New York, NY, pp. 439-474.
Koniger, M. and Winter, K. 1993. Growth and photosynthesis of Gossypium hirsutum L at high photon flux densities: effects of soil temperatures and nocturnal air temperatures. Agronomie 13: 423-431.
Korner, C. 1998. A re-assessment of high elevation treeline positions and their explanation. Oecologia 115: 445-459.
Krause, G.H., Cheesman, A.W., Winter, K., Krause, B. and Virgo, A. 2013. Thermal tolerance, net CO2 exchange and growth of a tropical tree species, Ficus insipida, cultivated at elevated daytime and nighttime temperatures. Journal of Plant Physiology 170: 822-827.
Kukla, G. and Karl, T.R. 1993. Nighttime warming and the greenhouse effect. Environmental Science and Technology 27: 1468-1474.
Kullmann, L. 1981. Pattern and process of present tree-limits in the Tarna region, southern Swedish Lapland. Fennia 169: 25-38.
Lange, O.L. and Lange, R. 1963. Untersuchungen uber Blattemperaturen, Transpiration und Hitzeresistenz an Pflanzen mediterraner Standorte (Costa Brava, Spanien). Flora 153: 387-425.
Laurance, W.F., Useche, D.C., Shoo, L.P., Herzog, S.K., Kessler, M., Escobar, F., Brehm, G., Axmacher, J.C., Chen, I-C., Arellano Gamez, L., Hietz, P., Fiedler, K., Pyrcz, T., Wolf, J., Merkord, C.L., Cardelus, C., Marshall, A.R., Ah-Peng, C., Aplet, G.H., del Coro Arizmendi, M., Baker, W.J., Barone, J., Bruehl, C.A., Bussmann, R.W., Cicuzza, D., Eilu, G., Favila, M.E., Hemp, A., Hemp, C., Homeier, J., Hurtado, J., Jankowski, J., Kattan, G., Kluge, J., Kroemer, T., Lees, D.C., Lehnert, M., Longino, J.T., Lovett, J., Martin, P.H., Patterson, B.D., Pearson, R.G., Peh, K.S-H., Richardson, B., Richardson, M., Samways, M.J., Senbeta, F., Smith, T.B., Utteridge, T.M.A., Watkins, J.E., Wilson, R., Williams, S.E. and Thomas, C.D. 2011. Global warming, elevational ranges and the vulnerability of tropical biota. Biological Conservation 144: 548-557.
Levitt, J. 1980. Responses of Plants to Environmental Stresses. Vol.1. Chilling, Freezing, and High Temperature Stresses. Academic Press, New York, NY.
Lewis, S.L., Phillips, O.L., Baker, T.R., Lloyd, J., Malhi, Y., Almeida, S., Higuchi, N., Laurance, W.F., Neill, D.A., Silva, J.N.M., Terborgh, J., Lezama, A.T., Vásquez Martinez, R., Brown, S., Chave, J., Kuebler, C., Núñez Vargas, P. and Vinceti, B. 2004. Concerted changes in tropical forest structure and dynamics: evidence from 50 South American long-term plots. Philosophical Transactions of the Royal Society of London Series B - Biological Sciences 359: 421-436.
Loehle, C. 1998. Height growth rate tradeoffs determine northern and southern range limits for trees. Journal of Biogeography 25: 735-742.
Long, S.P. 1991. Modification of the response of photosynthetic productivity to rising temperature by atmospheric CO2 concentrations: has its importance been underestimated? Plant, Cell and Environment 14: 729-739.
Lopatin, E. 2007. Long-term trends in height growth of Picea obovata and Pinus sylvestris during the past 100 years in Komi Republic (north-western Russia). Scandinavian Journal of Forest Research 22: 310-323.
Malhi, Y. and Phillips, O.L. 2004. Tropical forests and global atmospheric change: a synthesis. Philosophical Transactions of the Royal Society of London Series B - Biological Sciences 359: 549-555.
Morin, A. and Payette, S. 1984. Expansion recente du meleze a la limite des forets. (Quebec nordique). Canadian Journal of Botany 62: 1404-1408.
Nagashima, H., Yamano, T., Hikosaka, K. and Hirose, T. 2003. Effects of elevated CO2 on the size structure in even-aged monospecific stands of Chenopodium album. Global Change Biology 9: 619-629.
Overpeck, J.T., Bartlein, P.J. and Webb III, T. 1991. Potential magnitude of future vegetation change in eastern North America: Comparisons with the past. Science 254: 692-695.
Payette, S. 1974. Classification ecologique des formes de croissance de Picea glauca (Moench) Voss et de Picea mariana (Mill.) BSP. en milieux subarctiques et subalpins. Nat. Can. 101: 893-903.
Payette, S. and Filion, L. 1985. White spruce expansion at the tree line and recent climatic change. Canadian Journal of Forest Research 15: 241-251.
Perfors, T., Harte, J. and Alter, S.E. 2003. Enhanced growth of sagebrush (Artemisia tridentata) in response to manipulated ecosystem warming. Global Change Biology 9: 736-742.
Phillips, O.L., Baker, T.R., Arroyo, L., Higuchi, N., Killeen, T.J., Laurance, W.F., Lewis, S.L., Lloyd, J., Malhi, Y., Monteagudo, A., Neill, D.A., Núñez Vargas, P., Silva, J.N.M., Terborgh, J., Vásquez Martínez, R., Alexiades, M., Almeida, S., Brown, S., Chave, J., Comiskey, J.A., Czimczik, C.I., Di Fiore, A., Erwin, T., Kuebler, C., Laurance, S.G., Nascimento, H.E.M., Olivier, J., Palacios, W., Patiño, S., Pitman, N.C.A., Quesada, C.A., Saldias, M., Torres Lezama, A., B. and Vinceti, B. 2004. Pattern and process in Amazon tree turnover: 1976-2001. Philosophical Transactions of the Royal Society of London Series B - Biological Sciences 359: 381-407.
Root, T.L. and Schneider, S.H. 1993. Can large-scale climatic models be linked with multiscale ecological studies? Conservation Biology 7: 256-270.
Saleska, S.R., Shaw, M.R., Fischer, M.L., Dunne, J.A., Still, C.J., Holman, M.L. and Harte, J. 2002. Plant community composition mediates both large transient decline and predicted long-term recovery of soil carbon under climate warming. Global Biogeochemical Cycles 16: 10.1029/2001GB001573.
Searle, S.Y., Turnbull, M.H., Boelman, N.T., Schuster, W.S.F., Yakir, D. and Griffin, K.L. 2012. Urban environment of New York City promotes growth in northern red oak seedlings. Tree Physiology 32: 389-300.
Shiyatov, S.G. 1992. The upper timberline dynamics during the last 1100 years in the Polar Ural mountains. In: Frenzel, B. (Ed.) Oscillations of the Alpine and Polar Tree Limits in the Holocene. Fischer, Stuttgart, Germany, pp. 195-203.
Shiyatov, S.G. 2003. Rates of change in the upper treeline ecotone in the Polar Ural Mountains. Pages Newsletter 11: 8-10.
Stocklin, J. and Korner, C. 1999. Recruitment and mortality of Pinus sylvestris near the Nordic treeline: The role of climatic change and herbivory. Ecological Bulletin 47: 168-177.
Taub, D.R., Seeman, J.R. and Coleman, J.S. 2000. Growth in elevated CO2 protects photosynthesis against high-temperature damage. Plant, Cell and Environment 23: 649-656.
Turnbull, M.H., Murthy, R. and Griffin, K.L. 2002. The relative impacts of daytime and nighttime warming on photosynthetic capacity in Populus deltoides. Plant, Cell and Environment 25: 1729-1737.
Veres, J.S. and Williams III, G.J. 1984. Time course of photosynthetic temperature acclimation in Carex eleocharis Bailey. Plant, Cell and Environment 7: 545-547.
Volk, T. 1987. Feedbacks between weathering and atmospheric CO2 over the last 100 million years. American Journal of Science 287: 763-779.
Wang, J., Duan, B. and Zhang, Y. 2012. Effects of experimental warming on growth, biomass allocation, and needle chemistry of Abies faxoniana in even-aged monospecific stands. Plant Ecology 213: 47-55.
Woodward, F.I. 1987. Climate and Plant Distribution. Cambridge University Press, Cambridge, UK.
Woodward, F.I. 1988. Temperature and the distribution of plant species and vegetation. In: Long, S.P. and Woodward, F.I. (Eds.), Plants and Temperature. Vol. 42, The Company of Biologists Limited, Cambridge, UK, pp. 59-75.
Woodwell, G.M. 1989. The warming of the industrialized middle latitudes 1985-2050: Causes and consequences. Climatic Change 15: 31-50.
Wright, S.J. 2010. The future of tropical forests. In: Ostfeld, R.S. and Schlesinger, W.H. (Eds.). The Year in Ecology and Conservation Biology 2010. Wiley-Blackwell, Malden, Massachusetts, USA, pp. 1-27.
Wright, S.J., Muller-Landau, H.C. and Schipper, J. 2009. The future of tropical species on a warmer planet. Conservation Biology 23: 1418-1426.
Zelazowski, P., Malhi, Y., Huntingford, C., Sitch, S. and Fisher, J.B. 2011. Changes in the potential distribution of humid tropical forests on a warmer planet. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences 369: 137-160.
Last updated 29 January 2015



