By examining various properties of tree rings, researchers can deduce how historical increases in the air's CO2 concentration have already affected tree productivity and water use efficiency. Duquesnay et al. (1998) , for example, analyzed the relative amounts of 12C and 13C present in yearly growth rings of beech trees raised in silviculture regimes in northeastern France, which enabled them to discover that their intrinsic water use efficiencies rose by approximately 33% during the prior century, as the atmosphere's CO2 concentration rose from approximately 280 to 360 ppm. In another case, Rathgeber et al. (2000) used tree-ring density data to create an historical productivity baseline for forest stands of Pinus halepensis in southeastern France, from which they determined that the net productivity of such forests would likely increase by 8 to 55% with a doubling of the air's CO2 content.
One year earlier, when running a forest growth model, but one that was based on empirical observations reported in the literature, Lloyd (1999) determined that the rise in the atmospheric CO2 concentration since the onset of the Industrial Revolution had likely increased the net primary productivity of mature temperate deciduous forests by about 7%. In addition, he determined that a proportional increase in anthropogenic nitrogen deposition likely increased forest net primary productivity by 25%. And when he combined the two effects, the net primary productivity stimulation rose to 40%, which due to synergetic interactions was actually more than the sum of the growth enhancements resulting from the individual increases in CO2 and nitrogen acting by themselves.
Contemporaneously, Medlyn et al. (1999) had conducted a meta-analysis of data obtained from 15 atmospheric CO2 enrichment studies of European forest species growing in field environments, in order to determine their overall photosynthetic response to elevated (approximately doubled) atmospheric CO2 concentrations. And the resulting meta-analysis of the 21 researchers indicated that the twice-ambient CO2 concentrations utilized in the studies were found to have stimulated the trees' net photosynthetic rates by an average of 51%.
In a more recent study, Bragg et al. (2013) write that increasing CO2 concentrations "would be expected to favor trees over grasses by increasing the growth rates of trees (C3 plants) relative to tropical grasses (C4 plants), which are less responsive to CO2 (Ehleringer et al., 1997), and more generally by allowing faster-growing tree seedlings to escape the 'fire trap' in fire-prone grasslands and savannas (Bond and Midgley, 2000; Bond et al., 2003; Kgope et al., 2010)." And they say that "the large (~ 100 ppm) increase in CO2 concentration over the last glacial-interglacial transition has also been proposed as a major cause of the increase in global forest cover shown by pollen records (Street-Perrott et al., 1997; Archer et al., 1995, 2001; Bond and Midgely, 2000; MacInnis-Ng et al., 2011)."
To further investigate this phenomenon, which is detectable in the δ13C of leaf-wax biomarkers, Bragg et al. extracted such biomarkers from sediment cores collected along an offshore South Atlantic transect, in order to derive a record of vegetation changes in subequatorial Africa. In doing so the seven scientists report that their data suggested "a large increase in C3 relative to C4 plant dominance after the Last Glacial Maximum." And "using a process-based biogeography model that explicitly simulates 13C discrimination," they showed that "precipitation and temperature changes cannot explain the observed shift in δ13C values," which led them to conclude that "the physiological effect of increasing CO2 concentration is decisive, altering the C3/C4 balance and bringing the simulated and observed δ13C values into line."
In further discussing their findings, Bragg et al. say their results "have implications for the interpretation of the trend towards increased tree density in savannas, known as 'woody thickening'," noting that "an increase of CO2 concentration from 280 to 380 ppm should have increased the potential growth rates of C3 plants during the industrial era by ~ 15 to 20%." And they add that "a continued increase from 380 ppm to 550 ppm should cause a further increase of similar magnitude in the potential growth rates of C3 plants," which increase "would be expected to increase the competitive ability of C3 plants" and "further increase woody plant cover today, just as it did during the worldwide reforestation after the Last Glacial Maximum."
The results of these several studies demonstrate the fact that historic increases in the air's CO2 content must have already conferred great benefits upon earth's forests. But will future increases in the air's CO2 concentration do the same? Several research teams have embarked on long-term studies of various forest communities in an attempt to address this important question; and what follows are some the things that have been learned from their efforts.
Back in 1996, circular Free-Air CO2 Enrichment or FACE plots (30-m diameter) maintained at atmospheric CO2 concentrations of 360 and 560 ppm were established in a 15-year-old loblolly pine (Pinus taeda) plantation in North Carolina, USA, in order to study the effects of elevated CO2 on the growth and productivity of this particular forest community, which also had several hardwood species present in its understory. And based on some of the first sets of data to come out of this endeavor, Hymus et al. (1999) reported that net photosynthetic rates of the CO2-enriched loblolly pines trees were 65% greater than rates observed in control trees exposed to ambient air. These greater rates of carbon fixation contributed to the 24% greater growth rates observed in the CO2-enriched pine trees in the first year of this long-term study, according to Naidu and DeLucia (1999). In addition, DeLucia and Thomas (2000) reported that the elevated CO2 increased rates of net photosynthesis by 50 to 160% in four subdominant hardwood species present in the forest understory. Moreover, for one species - sweetgum (Liquidambar styraciflua) - the extra CO2 enhanced rates of net photosynthesis in sun and shade leaves by 166 and 68%, respectively, even when the trees were naturally subjected to summer seasonal stresses imposed by high temperature and low soil water availability, as reported by Herrick and Thomas (1999). And so it was that after two years of atmospheric CO2 enrichment, total ecosystem net primary productivity in the CO2-enriched plots was found to be 25% greater than what was measured in control plots fumigated with ambient air.
In a similar large-scale study, circular (25-m diameter) FACE plots maintained at atmospheric CO2 concentrations of 400 and 530 ppm were constructed within a ten-year-old sweetgum plantation in Tennessee, USA, in order to study the effects of elevated CO2 on the growth and productivity of this forest community. And after two years of treatment, Norby et al. (2001) reported that the modest 35% increase in the air's CO2 content boosted tree biomass production by an average of 24%. In addition, Wullschleger and Norby (2001) noted that the CO2-enriched trees displayed rates of transpirational water loss that were approximately 10% lower than those exhibited by control trees growing in ambient air; and as a result, elevated CO2 enhanced seasonal water use efficiencies of the mature sweetgum trees by 28 to 35%.
On a smaller scale, Pritchard et al. (2001) constructed idealized ecosystems (containing five different species) that were representative of regenerating longleaf pine (Pinus palustris Mill.) communities of the southeastern United States, fumigating them for 18 months with air containing 365 and 720 ppm CO2 in order to study the effects of elevated CO2 on this forest community. In so doing, they discovered that elevated CO2 increased the above- and below-ground biomass of the dominant longleaf pine individuals by 20 and 62%, respectively, while at the ecosystem level, elevated CO2 stimulated total aboveground biomass production by an average of 35%. And similar results for regenerating temperate forest communities were reported by Berntson and Bazzaz (1998), who documented a 31% increase in Transition Hardwood-White Pine-Hemlock forest mesocosm biomass in response to two years of fumigation with twice-ambient concentrations of atmospheric CO2.
Near the turn of the century, therefore, it was becoming quite clear that as the atmosphere's CO2 concentration continued to rise, forests would likely respond by exhibiting significant increases in biomass production, with the result that they would likely grow much more robustly and significantly expand their ranges, as was already being documented in many parts of the world, including Kansas, USA (Knight et al., 1994) and the Budal and Sjodal valleys of Norway (Olsson et al., 2000).
In the first of these cases, aerial photographs taken over a 46-year time period were used to analyze the dynamics and spatial extent of gallery forest on the Konza Prairie Research Natural Area (KPRNA) in Kansas, USA, between 1939 and 1985. This work revealed that over the 46-year period of study, total gallery forest area increased from 157 hectares to 241 hectares, while going further back in time and studying additional historical information obtained from the Original Land Office Surveys of KPRNA, it became evident that total forest area in this region increased fully 97% between 1859 and 1939, leading Knight et al. to conclude that there was "no question that the absolute amount of forested areas has increased."
Taking an even longer view of the subject, it should be noted that the explorer Coronado in 1541 stated, in reference to the Great Plains of America, that "there is not any kind of wood in all these plains, away from the gullies and rivers, which are very few." Clearly, therefore, a dramatic increase in forest growth has certainly occurred in this region since that time, and especially over the last century and a half. And one of the reasons for this increase is most certainly the historical increase in the earth's atmospheric CO2 concentration. Rising from a value of 265 ppm at the time of Coronado, to a value of 370 ppm in 1994, the increased CO2 had a pronounced positive impact on the photosynthesis and growth of woody species on every continent of the globe where trees are found, as has been further elucidated by Idso (1995).
In Norway, in the second of the cases mentioned above, mountains have been used for supporting domestic livestock for the past 4,000 years, but especially since the 16th century, which saw the development of the summer farming system there. Olsson et al. (2000) thus speculated that this activity originally reduced forested areas, but that changes in farming practices had more recently been allowing the forests to return. In investigating this hypothesis, they therefore studied two valleys - Budal and Sjodal - in Mid-Norway, which they said were representative of core areas of the Norwegian summer farming ecosystems that were "shaped by human activities rooted in pre-history." More specifically, they analyzed changes in land use and landscape patterns in the two areas over the period 1960-1993; and this work revealed that grasslands and heathlands that had long dominated the mountain slopes of the two study areas were, in their words, "today decreasing due to forest invasion," which they said was characterized by "the spread of subalpine woodlands, and a raised treeline."
Olsson et al. thus concluded that the expansion of the subalpine Norwegian woodlands was "primarily related to changes in the human use of those areas," which in their estimation were "much more influential than possible effects of climate change." However, it is also possible that the concurrent rise in the atmosphere's CO2 concentration may have played a role in the forests' comeback as well. In any event, the ongoing increase in the presence of forests in the mountain valleys of Norway is but one more manifestation of the spreading of woody species over the face of the planet that is helping to slow the rate of rise of the atmosphere's CO2 concentration.
In further studying this important subject, Walker et al. (2000) grew ponderosa pine (Pinus ponderosa Dougl.) seedlings for five years in open-top chambers having atmospheric CO2 concentrations of 350, 525, and 700 ppm on soils of low, medium and high nitrogen content in order to determine the interactive effects of these two variables on the long-term growth response of this particular tree species. In doing so, they found that the moderate level of atmospheric CO2 enrichment (525 ppm) had the greatest effect on tree height and trunk diameter in the first three years of the experiment. However, by the time years four and five were reached, trees grown at 700 ppm CO2 exhibited the greatest growth responses to elevated CO2. At final harvest, for example, the trees exposed to twice-ambient levels of atmospheric CO2 had heights that were 43, 64 and 25% greater than those of trees exposed to ambient air and conditions of high, medium and low levels of soil nitrogen, respectively. And in similar manner, trunk diameters of trees fumigated with 700 ppm CO2 for five years were 24, 73, and 20% greater than trunk diameters of ambiently-grown trees exposed to high, medium and low levels of soil nitrogen. As a result, one could expect that regenerating forests of ponderosa pine will likely display significant increases in growth, as the CO2 content of the air continues its upward trajectory.
In a contemporary study, Naumburg and Ellsworth (2000) measured photosynthetic rates in leaves of four hardwood saplings growing beneath the canopy of a Pinus taeda forest, several portions of which were exposed to either ambient or enriched (ambient + 200 ppm) atmospheric CO2 concentrations in a FACE study spanning two complete years. The measurements were made under conditions of both low and high light intensity, which commonly exist beneath maturing forest canopies due to shading and intermittent illumination by sunflecks, respectively. Thus, the two researchers studied the effects of elevated CO2 on sapling performance under the variable light conditions that prevail beneath the canopies of real-world forests.
The data thereby collected indicated that the elevated CO2 increased the mean photosynthetic rates of four hardwood understory saplings by 60 and 40% under high and low light conditions, respectively. Also, in going from shaded to lighted conditions, elevated CO2 had no effect on photosynthetic induction, with ambient and CO2-enriched species both reaching 90% of their maximal transient photosynthetic rates at approximately the same time. However, in going from lighted to shaded conditions, elevated CO2 extended the time during which maximal rates of photosynthesis were maintained. Thus, elevated CO2 slowed the rate of photosynthetic decline caused by the onset of shading; and as a result, the shaded leaves of CO2-enriched saplings maintained greater rates of photosynthesis for longer periods of time than did shaded leaves of saplings growing in ambient air, which allowed the CO2-enriched leaves to sequester greater amounts of carbon than was expected from photosynthetic measurements made under steady-state conditions.
As the air's CO2 content continues to rise, therefore, saplings growing beneath the canopies of larger trees will likely increase their rates of photosynthesis under both high and low light conditions characteristic of intermittent shading and illumination by sunflecks. Moreover, because elevated CO2 concentrations allow saplings to maintain higher rates of photosynthesis for longer periods of time when going from lighted to shaded conditions, such trees should be able to sequester greater quantities of carbon than they do now. So powerful is this phenomenon, in fact, the two researchers stated that current estimates of the enhancement of long-term carbon gains by forests under conditions of elevated atmospheric CO2 "could be underestimated by steady-state photosynthetic measures."
Around this same time, Hamilton et al. (2001) described the short- and long-term respiratory responses of loblolly pine (Pinus taeda) and sweetgum (Liquidambar styraciflua) trees to the ambient and elevated atmospheric CO2 concentrations (360 and 560 ppm) of the 30-meter-diameter FACE plots of the long-running loblolly pine plantation experiment in North Carolina, USA, where the deciduous trees had naturally established themselves beneath the primarily coniferous canopy, after which they reported that the modest 200-ppm increase in atmospheric CO2 concentration resulted in no significant short-term suppression of dark respiration rates in needles of loblolly pine. It did, however, reduce rates of dark respiration in sweetgum leaves by an average of 10%. The long-term exposure to elevated CO2 also did not appear to alter maintenance respiration, which is the amount of CO2 that is needed to maintain existing tissue, in either of the tested species. But growth respiration, which is the amount of CO2 respired when constructing new tissues, was reduced by 21% in loblolly pine and 39% in sweetgum leaves that reached the top of the canopy. Thus, as the air's CO2 content continues to rise, it is likely that these two forest species will exhibit increased rates of photosynthesis that will provide them with more of the raw materials required for constructing new and greater amounts of biomass; while at the same time, the costs of respiration during the synthesis of new tissues likely will be reduced, thus allowing greater amounts of carbon to be retained in the trees and thereby helping to reduce the rate of rise of the atmosphere's CO2 concentration.
Working at the same location, Hussain et al. (2001) collected seeds from trees exposed to both atmospheric CO2 concentrations in order to study the effects of elevated CO2 on seed characteristics, germination success and early seedling growth. This effort revealed that seeds collected from CO2-enriched trees were 91% heavier than seeds collected from trees growing in ambient air. In addition, the CO2-enriched seeds had a lipid content that was 265% greater than that observed in seeds produced on the ambient-treatment trees; and the germination success for seeds developed under atmospheric CO2 enrichment was more than three times greater than that observed for control seeds developed at ambient CO2, regardless of germination CO2 concentration. Moreover, the seeds from the CO2-enriched trees germinated approximately five days earlier than their ambiently-produced counterparts, again regardless of germination CO2 concentration. And seedlings developing from seeds collected from CO2-enriched trees displayed significantly greater root lengths and needle numbers than seedlings developing from trees exposed to ambient air, also regardless of current growth CO2 concentration. Thus, as the CO2 content of the air continues to increase, loblolly pine trees will likely display significant increases in their photosynthetic rates; and the enhanced carbohydrate supplies resulting from this phenomenon will likely be used to increase seed weight and lipid content. These seeds should consequently exhibit significant increases in germination success; and their enhanced lipid content will likely lead to greater root lengths and needle numbers in developing seedlings. Thus, when these seedlings become photosynthetically-active, they will likely photosynthesize and produce biomass at greater rates than those currently exhibited by seedlings growing under ambient CO2 concentrations, in a positive cycle that keeps repeating itself.
One year later, Hamilton et al. (2002) reported what they had learned from the Duke Forest FACE Experiment over a period of four years. This ecosystem - a predominantly loblolly pine (Pinus taeda L.) forest with sweetgum (Liquidambar styraciflua L.) and yellow poplar (Liriodendron tulipifera L.) trees as sub-dominants, together with numerous other trees, shrubs and vines - was established in 1983 following the clear-cutting of a regenerating forest in 1979. The experiment was begun in August of 1996, when three 30-meter-diameter FACE plots were enriched with CO2 to atmospheric concentrations 200 ppm above ambient, while three similar plots were maintained at ambient conditions as controls. And based on the standing pool of ecosystem biomass in 1998 and more recent measurements of various carbon fluxes, they calculated a complete carbon budget for the forest for that particular year. This exercise revealed that the extra CO2 supplied to the FACE plots stimulated net ecosystem productivity (NEP) by 41%, while for a 300-ppm increase in atmospheric CO2 concentration - which is the most common increment of CO2 enrichment that has been employed in CO2 enrichment experiments over the years - this result translates into a CO2-induced NEP increase on the order of 60%, which represents a significant stimulation of biological carbon sequestration, especially for trees growing on a soil the researchers described as being of "low nitrogen and phosphorus availability."
Publishing simultaneously, Norby et al. (2002) described how a FACE study was established within a ten-year-old stand of sweetgum (Liquidambar styraciflua L.) trees growing in a forest plantation on nutrient-rich soils in Tennessee, USA, where the trees were exposed to atmospheric CO2 concentrations of 360 and 550 ppm. Due to that CO2 increase, ecosystem net primary productivity rose by 21% in all three years of their study, while aboveground woody biomass rose by 33% in the first year, 15% in the second year and 7% in the third year, while net primary productivity remained unchanged. The reason for the drop was that an increasing amount of newly-fixed carbon in the CO2-enriched trees was being utilized to increase fine-root and leaf production in each progressive year. But over the three-year period of their study, 77% of the additional fixed carbon was nevertheless allocated to aboveground woody biomass, leading the eleven researchers to conclude that "this experiment has provided the first evidence that CO2 enrichment can increase productivity in a closed-canopy deciduous forest."
In providing background information for another paper from the same year, Aidar et al. (2002) noted that the leguminous Hymenaea courbaril L. tree - which is commonly known as jatoba and grows to a height of 20-30 meters with a trunk diameter of 200 cm - is "a late secondary/climax species that is one of the most important trees in mature tropical forests of the Americas," noting further that it occurs "in more than 30% of 43 inventories made in the extra-Amazonian riparian forests (Rodrigues and Nave, 2000)" and that it shows "wide distribution in [the] neotropics, from the Caribbean isles, Mexico and Peru to Southeastern Brazil (Allen and Allen, 1981)."
That being said, the six scientists sprouted and grew jatoba seedlings in pots placed within small open-top chambers maintained at atmospheric CO2 concentrations of 360 and 720 ppm within a shaded glasshouse (to simulate the low light regime at the forest floor where the seeds typically germinate) for a period of 70 days, over which time they measured rates of net photosynthesis in seedlings with and without cotyledons, which they removed from half of the plants. These efforts revealed a persistent 2-fold increase in photosynthesis both with and without cotyledons, when the seedlings were exposed to elevated CO2. In addition, they observed a 35% increase in the water use efficiency of the seedlings. And as a result, Aidar et al. stated that "under the climatic conditions forecasted on the basis of the present carbon dioxide emissions, Hymenaea courbaril should establish faster in its natural environment and might also serve as an efficient mechanism of carbon sequestration within the forest." They also opined that the CO2-induced increase in water use efficiency may enable jatoba "to tolerate dryer and more open environments, which should allow them to better cope with drought stress or a more seasonal climate." Last of all, they stated that the jatoba tree would likely exhibit similar positive responses to even greater emissions of CO2, for they noted that light-saturated photosynthesis in jatoba seedlings continued to rise in response to increasing atmospheric CO2 concentrations well above 1,000 ppm. What is more, they reported that they "have measured the saturation level of some other tropical trees from the rain forest (Caesalpinia echinata, Piptadenia gonoacantha, Tibouchina granulose, T. pulchra) and all of them [also] saturate at relatively high CO2 concentrations." Hence, it is quite likely that neotropical forests in general are suited to much higher-than-present atmospheric CO2 concentrations and would fare far better than they do today in a CO2-enriched world of the future.
One year later, Rathgeber et al. (2003) used tree-ring width and density chronologies (both earlywood and latewood) from 21 stands of Aleppo pine (Pinus halepensis Mill.) in the Provence region of southeast France to calibrate the BIOME3 biogeochemistry model of forest productivity in terms of growth responses to known historical changes in atmospheric temperature, precipitation and CO2 concentration, after which the BIOME3 model was used to calculate changes in the mean productivity of the same forest stands that could be expected to result from changes in these parameters driven by a doubling of the air's CO2 content, as calculated by Meteo-France's ARPEGE atmospheric general circulation model when downscaled to that specific part of the country. This work revealed that in response to the predicted changes in climate, forest productivity increased moderately for all stands (17% to 24%); while in response to the aerial fertilization effect of the doubling of the atmosphere's CO2 concentration, it increased considerably more (72% to 86%). Even more impressively, when the climatic changes and atmospheric CO2 increase were considered together, forest productivity increased still more (107% to 141%), which response is greater than that provided by the sum of their individual contributions, due to the amplifying synergy that exists among these factors with respect to their combined impact on basic plant physiological processes.
Also in the same year, and also from out of Europe, came the study of Bergh et al. (2003), who used a boreal version of a process-based simulation model (BIOMASS) to quantify the individual and combined effects of elevated air temperature (2 and 4°C above ambient) and CO2 concentration (350 ppm above ambient) on the net primary production (NPP) of both coniferous (Pinus sylvestris, Picea abies) and deciduous broad-leaf (Fagus sylvatica, Populus trichocarpa) forests growing in Denmark, Finland, Iceland, Norway and Sweden. For a group of three of the four species (P. sylvestris, P. abies, P. trichocarpa), air temperature increases of 2 and 4°C led to mean NPP increases of 11 and 20%, respectively; while for the other species (F. sylvatica) there were corresponding 21 and 48% decreases in NPP. However, when the atmosphere's CO2 concentration was simultaneously increased from 350 to 700 ppm, the corresponding mean NPP increases of the three-species group rose to 41 and 55%; while the NPP of F. sylvatica jumped from -21 and -48% to +37 and +10%. Last of all, when the atmosphere's CO2 content was doubled at the prevailing ambient air temperature, the mean NPP value of the three-species group rose by 27%, while that of F. sylvatica rose by 58%. Consequently, as the air's CO2 content continues to climb, the major tree species of Denmark, Finland, Iceland, Norway and Sweden should become significantly more productive; and if air temperature also rises, most of them will grow even better.
In another study from the same year that was conducted at the Poplar Free Air CO2 Enrichment (PopFACE) facility described by Miglietta et al. (2001), which is located near Viterbo in Central Italy, Bernacchi et al. (2003) worked with three hybrid poplars that they described as "so fast growing that they provide a rare opportunity to grow a plantation forest from planting to canopy closure of tall trees (greater than 9 m) in just 3 years." This was done in a field previously used for wheat cultivation that was planted with the hybrid Populus x euramericana Dode (Guinier) - (P. deltoides Bart. ex Marsh. x P. nigra L., I-214) - with the exception of six 30 x 30-m square plots that each contained a 22-m-diameter FACE ring. Three of these rings were maintained at 370 ppm CO2, while the other three were maintained at 550 ppm CO2. Within each of them were grown equal-area sections of P. alba L. (genotype 2AS1), P. nigra L. (genotype Jean Pourtet) and P. x euramericana (genotype I-214), which were maintained free of drought by a drip irrigation system, while periodic measurements of net photosynthesis and stomatal conductance were made over the three-year period of growth from the seedling to closed-canopy forest stage.
As for their findings, there was no response of leaf stomatal conductance to atmospheric CO2 enrichment. In the case of net photosynthesis, however, the team of seven scientists observed a 38% increase in light-saturated net photosynthesis at 25°C, which they described as being "close to the maximum theoretically possible" in response to the 49% increase in atmospheric CO2 concentration employed in their study. Daily integrated rates of in situ photosynthesis were even higher, rising by 40% to almost 90% (which is approximately equivalent to 150% in response to a 300-ppm increase in the air's CO2 concentration), due to the fact, in the words of Bernacchi et al., that "daytime leaf temperatures were typically over 30°C resulting in a larger stimulation of leaf photosynthesis by elevated CO2 than would be evident at 25°C (Long, 1991)." And this huge stimulation of daily net photosynthesis illustrates the enormous potential for earth's trees, even in closed-canopy forests, to positively respond to the ongoing rise in the air's CO2 content.
Inching ahead another year, Su and Sang (2004) used an ecosystem process model, BIOME-BGC, to explore the sensitivity of the net primary productivity (NPP) of an oak (Quercus liaotungensis Koidz) forest ecosystem in the Beijing area of China to global climate changes projected to be caused by rising atmospheric CO2 concentrations. And what did they find? For a doubling of the air's CO2 concentration from 355 to 710 ppm, the Beijing oak forest's NPP was calculated to rise by 14.0%, while with a concomitant temperature increase of 2°C, its NPP was calculated to rise by 15.7%; and with an additional 20% increase in precipitation, it was calculated to rise by 25.7%. Last of all, with a 20% increase in precipitation and a 4°C increase in temperature, it was calculated to rise by 25.7%. So what are the implications of these findings?
In contrast to typical climate-alarmist claims, many projections of ecosystem responses to potential environmental change are not catastrophically negative, even when the increases in air temperature they employ are unrealistically large, such as the 4°C rise employed here. In fact, as in this particular case, many of the responses are actually positive, and strongly so. One of the reasons for this discrepancy is that climate-alarmists typically downplay, or even disregard, the many mitigating effects of concomitant atmospheric CO2 enrichment (which they claim is the driver for climate change and therefore cannot be ignored), including the ability of elevated levels of atmospheric CO2 to (1) dramatically increase plant growth, (2) significantly reduce plant water loss by transpiration and thereby (3) greatly enhance plant water use efficiency, and (4) actually alter the physiology of plants to where they prefer warmer temperatures, which phenomenon is expressed by an increase in the temperature at which plants photosynthesize most efficiently. Any projections of ecosystem responses to potential climate change, and especially those that assume the rising CO2 content of the atmosphere is their cause, must include these very real phenomena. And when they are included, the results are often positive, as was the case in this study.
Backtracking just a bit, in a small booklet published by the University of Minnesota (USA) nearly two decades ago, Idso (1995) laid out the evidence for a worldwide increase in the growth rates of earth's forests that had been coeval with the progression of the Industrial Revolution and the rising CO2 content of the atmosphere. The development of this concept began with the study of LaMarche et al. (1984), who (1) analyzed annual growth rings of two species of pine tree growing near the timberline in California, Colorado, Nevada and New Mexico, and who in doing so (2) discovered large increases in growth rate between 1859 and 1983, which exceeded what might have been expected from climatic trends, but which were consistent with the global trend of atmospheric CO2.
The next stage of the overarching developmental journey was inspired by a study of ring-width measurements of Douglas fir trees in British Columbia, Canada, which also revealed a marked increase in the growth rates of the trees' in later decades (Parker et al., 1987), and which led the principal investigator of the project to state that "environmental influences other than increased CO2 have not been found that would explain this [phenomenon]." West (1988) reported much the same thing with respect to long-leaf pines in Georgia, i.e., that their annual growth increments had begun to rise at an unusual rate about 1920, increasing by approximately 30% by the mid-1980s; and he too stated that "the increased growth cannot be explained by trends in precipitation, temperature, or Palmer Drought Severity Index," leaving the rising CO2 content of the atmosphere as the likely cause of the observed increase in productivity.
Contemporaneously, stands of Scots pines in northern Finland were found to have experienced growth increases ranging from 15 to 43% between 1950 and 1983 (Hari et al., 1984; Hari and Arovaara, 1988). As for the cause of this phenomenon, the researchers who had been involved in the work stated that "CO2 seems to be the only environmental factor that has been changing systematically during this century in the remote area under study," and it was thus to this factor that they looked for an explanation of their observations.
The next major development in the continuing saga was the finding of Graybill and Idso (1993) that very long ring-width chronologies (some stretching back nearly 1800 years) of high-altitude long-lived bristlecone, foxtail and limber pine trees in Arizona, California, Colorado and Nevada all developed an unprecedented upward growth trend somewhere in the 1850s that continued as far towards the present as the records extended. In this case, too, like the ones that preceded it, comparisons of the chronologies with temperature and precipitation records ruled out the possibility that either of these climatic variables played a significant role in enhancing the trees' growth rates, strongly implicating the historical rise in the air's CO2 concentration as the factor responsible for their ever-increasing productivity over the prior century and a half.
Also working in 1993, West et al. developed tree-ring chronologies for both "young" (96-136 years of age, mean = 125 years) and old (161-396 years of age, mean = 250 years) longleaf pine (Pinus palustris Mill.) trees in one of the last remaining old-growth stands of this species located in Thomas County, Georgia. The authors report that "the expected annual ring-width increment of undisturbed trees is a negative exponential function with time," but they report that the trees in their study "began a positive departure from this trend approximately 30-50 years ago," i.e., prior to 1987, and that "the beginning of the positive response began in the 1920s for many individual trees," so that trees at the end of their study (in 1987) were reaching larger sizes more rapidly than did equally-old trees 50 years earlier. Determining that climate variables were "deficient in explaining the growth trends observed," they suggested that "increased atmospheric CO2 is a possible explanation for initiation of the observed trend."
Perhaps the most striking evidence of all for the significant growth enhancement of earth's forests being driven by the historical increase in the air's CO2 concentration was provided by the study of Phillips and Gentry (1994). Noting that turnover rates of mature tropical forests correlate well with measures of net productivity (Weaver and Murphy, 1990), the two scientists assessed the turnover rates of 40 tropical forests from around the world in order to test the hypothesis that global forest productivity was increasing in situ; and in doing so, they found that the turnover rates of these highly productive forests had indeed been rising ever higher since at least 1960, with an apparent pan-tropical acceleration since 1980. In discussing what might have been causing this phenomenon, they stated that "the accelerating increase in turnover coincides with an accelerating buildup of CO2," and as Pimm and Sugden (1994) stated in a companion article, it was "the consistency and simultaneity of the changes on several continents that lead Phillips and Gentry to their conclusion that enhanced productivity induced by increased CO2 is the most plausible candidate for the cause of the increased turnover."
Four years later, Phillips et al. (1998) reported another impressive finding. Working with data on tree basal area (a surrogate for tropical forest biomass) for the period 1958-1996, which they obtained from several hundred plots of mature tropical trees scattered about the world, they found that the average forest biomass for the tropics as a whole had increased substantially. In fact, they calculated that the increase amounted to approximately 40% of the missing terrestrial carbon sink of the entire globe. Hence, they suggested that "intact forests may be helping to buffer the rate of increase in atmospheric CO2, thereby reducing the impacts of global climate change," as Idso (1991a,b) had earlier suggested; and they identified the aerial fertilization effect of the increasing CO2 concentration of the atmosphere as one of the primary factors responsible for this phenomenon. Other contemporary studies also supported their findings (Grace et al., 1995; Malhi et al., 1998), verifying the fact that neotropical forests were indeed accumulating ever more carbon at ever-increasing rates. And Phillips et al. (2002) subsequently wrote that this phenomenon was occurring "possibly in response to the increasing atmospheric concentrations of carbon dioxide (Prentice et al., 2001; Malhi and Grace, 2000)."
More recently, Laurance et al. (2004a) reported accelerated growth in the 1990s relative to the 1980s for the large majority (87%) of tree genera in 18 one-hectare plots spanning an area of about 300 km2 in central Amazonia, while Laurance et al. (2004b) observed similarly accelerated tree community dynamics in the 1990s relative to the 1980s. And once again it was suggested, in the words of Laurance et al. (2005), that these "pervasive changes in central Amazonian tree communities were most likely caused by global- or regional-scale drivers, such as increasing atmospheric CO2 concentrations (Laurance et al., 2004a,b)."
Expanding upon this theme, Laurance et al. (2005) interpreted the observed changes as "being consistent with an ecological 'signature' expected from increasing forest productivity (cf., Phillips and Gentry, 1994; Lewis et al. 2004a,b; Phillips et al., 2004)." But they noted that they had been challenged in this conclusion by Nelson (2005); and they thus went on to consider his arguments in some detail, methodically dismantling them one by one.
As time progressed, however, it became less and less popular for scientists to report positive consequences of rising atmospheric CO2 concentrations, due to pressures spawned by various publications of the IPCC and their politically-correct interpreters. And as a result, the findings of Phillips and company began to be repeatedly questioned (Sheil, 1995; Sheil and May, 1996; Condit, 1997; Clark, 2002; Clark et al., 2003). But in response to these several challenges to their work, Phillips and 17 other like-minded researchers (Lewis et al., 2005), including one scientist who had earlier criticized the group's conclusions, published a new analysis that vindicated their earlier results.
One of the primary criticisms of Phillips et al.'s findings was the fact that their meta-analyses included sites with a wide range of tree census intervals (2-38 years), which critics contended could be confounding. However, in their detailed study of this potential problem, Lewis et al. (2005) found that a re-analysis of Phillips et al.'s published results showed that the pan-tropical increase in turnover rates over the late 20th century "cannot be attributed to combining data with differing census intervals." Or as they stated in another place, "the conclusion that turnover rates have increased in tropical forests over the late 20th century is robust to the charge that this is an artifact due to the combination of data that vary in census interval (cf. Sheil, 1995)."
Lewis et al. (2005) additionally noted that "Sheil's (1995) original critique of the evidence for increasing turnover over the late 20th century also suggests that the apparent increase could be explained by a single event, the 1982-83 El Niño Southern Oscillation (ENSO), as many of the recent data spanned this event." However, Lewis et al. correctly reported that "recent analyses from Amazonia have shown that growth, recruitment and mortality rates have simultaneously increased within the same plots over the 1980s and 1990s, as has net above-ground biomass, both in areas largely unaffected, and in those strongly affected, by ENSO events (Baker et al., 2004; Lewis et al., 2004a; Phillips et al., 2004)." And these developments continue to support the view that there has indeed been an increase in forest growth rates throughout the world that has gradually accelerated over the years in concert with the historical increase in the air's CO2 concentration.
Moving on, Hanson et al. (2005) used models that performed well in a multiyear simulation of the current carbon and water budgets of an upland-oak forest (Hanson et al., 2004) to evaluate the influence of single and multifactor environmental change scenarios projected for 2100, with and without modifications to account for physiological and growth responses learned from long-term field experimental studies (Winnett, 1998), where the environmental changes to be evaluated were a 385 ppm increase in CO2, a 20 ppb increase in O3, a 4°C increase in temperature, and a 20% increase in winter precipitation, and where the responses to those changes "were derived primarily from field experimental studies on deciduous trees and forest systems." And what did they learn?
Initial simplistic model projections of annual net ecosystem carbon exchange (NEEa) for the single-factor change scenarios yielded NEEa responses of +191% for CO2, -206% for temperature, 0% for precipitation, and -35% for O3, such that the combined influence of the four environmental changes yielded a 29% reduction in mean NEEa. However, as Hanson et al. reported, "when experimentally observed physiological adjustments were included in the simulations (e.g. acclimation of leaf respiration to warming), the combined influence of the year 2100 scenario resulted in a 20% increase in NEEa, not a decrease." In addition, they stated that "consistent with the annual model's predictions, simulations with a forest succession model run for gradually changing conditions from 2000 to 2100 indicated an 11% increase in stand wood biomass in the future compared with current conditions." Thus, even with the unrealistically extreme temperature change investigated in their study, which came from the IPCC's Third Assessment Report (Houghton et al., 2001) and the US National Assessment Synthesis Team's report on climate-change impacts (NAST, 2000), the knowledge that had been gained from real-world experiments demonstrated that desirable plant responses to atmospheric CO2 enrichment were sufficient to override the huge negative influence of the inflated warming and produce a significant enhancement in NEEa.
In a study published about this same time in the Proceedings of the National Academy of Sciences, another team of 19 researchers (Norby et al., 2005) stated that "experiments have unequivocally shown that plants can grow faster and larger in a CO2-enriched atmosphere, and the mechanisms of response are well understood." Furthermore, they stated that computer simulations of climatic responses to atmospheric CO2 "will be incorrect if the magnitude of the CO2 fertilization effect is not represented accurately." Hence, to help overcome this deficiency (which is but one of many inherent in even the most advanced of today's climate models), they provided an analysis of the net primary productivity (NPP) response of closed-canopy forests to increases in the air's CO2 concentration in the only Free-Air CO2 Enrichment (FACE) studies that had been conducted on assemblages of trees that were large enough and spatially concentrated enough to meet this important criterion of realism.
The four multi-year experiments Norby et al. analyzed were: (1) the Duke-FACE study near Durham, North Carolina, USA, which was initiated in an established monoculture plantation of evergreen loblolly pine (Pinus taeda) trees, (2) the ORNL-FACE study near Oak Ridge, Tennessee, USA, which was initiated in an established monoculture of deciduous sweetgum (Liquidambar styraciflua) trees, (3) the Aspen-FACE study near Rhinelander, Wisconsin, USA, which was initiated on bare ground but was ultimately comprised of multi-tree assemblages dominated by Populus species, and (4) the POP-EUROFACE study near Tuscania (Viterbo), Italy, which was also initiated on bare ground and ultimately comprised of multi-tree assemblages dominated by Populus species.
To be compatible with the first two experiments in terms of the trees' state of development, no data were used from the latter two experiments until the trees had grown to the point where their canopies were completely closed. Under these conditions, and across all appropriate years of all experiments (6 years in the Duke-FACE study, 5 years in the ORNL-FACE study, 1 and 3 years in different portions of the Aspen-FACE study, and 2 years in the POP-EUROFACE study), the average atmospheric CO2 concentration in the ambient-air control plots was 376 ppm, while the average concentration in the CO2-enriched plots was 550 ppm, yielding an average CO2 concentration differential of 174 ppm between the two CO2 treatments. So what was learned from this massive experimental enterprise?
In what the four groups of researchers describe as a "surprising consistency of response across diverse sites," they found that forest NPP was enhanced by 23 +/- 2% at the median NPP of their combined data set in response to the 174-ppm increase in the air's CO2 concentration. This NPP stimulation is substantial, considering that most of the CO2 stimulation figures one sees in the scientific literature are for a 300-ppm increase in atmospheric CO2 concentration. And linearly extrapolating Norby et al.'s median result to correspond to that greater CO2 concentration differential yields an NPP stimulation of approximately 40%, or just slightly less, due to the fact that as the air's CO2 content rises, the NPP stimulation provided by extra CO2 rises slightly more slowly.
In further commenting on their findings, Norby et al. indicated that the data in their analyses all came from "fast-growing, early successional stands, and there had been no evidence to date for a negative feedback on NPP through nitrogen availability in these stands," as some had suggested would occur. As a result, Norby et al. confidently concluded that "the effect of CO2 fertilization on forest NPP is now firmly established, at least for young stands in the temperate zone."
Nevertheless, nitrogen availability does play a role in this phenomenon. In the Duke-FACE study, for example, where Norby et al. say "a wide range of response to CO2 enrichment across replicate plots correlated with differences in soil nitrogen availability," it was observed that "under low nitrogen availability, CO2 enrichment increased NPP by 19%, whereas under intermediate and high nitrogen availability the percent CO2 stimulation was 27%," or 42% greater (27%/19% = 1.42). This observation is very important, for it is "almost certain," in the words of Shaw et al. (2002), that significant nitrogen deposition originating from anthropogenic activities will continue to accompany the ongoing rise in the atmosphere's CO2 concentration throughout the foreseeable future; and this phenomenon should further boost forest NPP. But by how much?
Looking to the past, Lloyd (1999) had calculated that from 1730 to the early 1980s the increase in temperate deciduous forest NPP due solely to the historical increase in the atmosphere's CO2 concentration was approximately 7%, and that the increase in NPP due to a modest proportional increase in nitrogen deposition over the same time period would have been about 25%. However, when CO2 and nitrogen increased together in the model employed by Lloyd, the NPP stimulation was 40%, which is even more than the sum of the individual contributions of the extra CO2 and nitrogen. Although this exercise does not allow for a precise prediction of the percentage stimulation of forest NPP in response to future concomitant increases in atmospheric CO2 content and nitrogen deposition, it does suggest that the increase will likely be significantly larger than what is suggested by the analysis of Norby et al., which deals solely with the effects of increasing CO2.
In another analysis of the subject, Lewis (2006) reported that over the prior two decades, intact tropical forests had exhibited "concerted changes in their ecology, becoming, on average, faster growing - more productive - and more dynamic, and showing a net increase in above-ground biomass," all of which rates of increase were greater than the previously documented increases in the rates of these phenomena. What is more, Lewis noted that "preliminary analyses also suggest the African and Australian forests are showing structural changes similar to South American forests."
What had been causing this suite of concerted changes? Lewis wrote that "the results appear to show a coherent fingerprint of increasing net primary productivity across tropical South America, caused by a long-term increase in resource availability (Lewis et al., 2004a,b)." So what "resources" might have been involved? Lewis gave four possibilities: increases in solar radiation, air temperature, nutrient deposition and atmospheric CO2 concentration. But after analyzing each of them in detail, he concluded that "the most parsimonious explanation is the increase in atmospheric CO2, because of the undisputed long-term historical increase in CO2 concentrations, the key role of CO2 in photosynthesis, and the demonstrated positive effects of CO2 fertilization on plant growth rates including experiments on whole temperate-forest stands (Ainsworth and Long, 2005)," or as he stated in another place in his review, the explanation resided in "the anthropogenic increase in atmospheric carbon dioxide concentrations, increasing forest net primary productivity leading to accelerated forest growth and dynamics."
In light of the voluminous and undeniable real-world observations reported by Lewis, it must be acknowledged that where tropical forests have not been decimated by the targeted and direct destructive actions of man, such as the felling and burning of trees, forest productivity has been growing ever greater with the passing of time, rising hand-in-hand with the increasing CO2 content of the air; and it has been doing so in spite of all concomitant changes in atmospheric, soil and water chemistry, as well as "dreaded" 20th-century global warming, which is claimed by climate alarmists to have been unprecedented over the past two millennia. Real-world evidence also suggests that we have the anthropogenic-induced increase in the air's CO2 content to thank for this beneficent state of affairs, which further suggests that if humanity will but cease its direct physical assaults upon earth's tropical forests, we should have nothing to fear about their future well-being but ill-founded fear itself, which could well drive us to irrationally deprive them of that which appears to have supported the phenomenal increase in productivity that they have experienced over the past half-century. Nevertheless, many people - including Lewis - believe that the forests' biological response to rising CO2 may saturate sometime in the future, and that the predicted climatic effects of anthropogenic CO2 emissions might ultimately overpower this positive effect and lead to a significant downturn in tropical forest productivity, once again highlighting the need to resolve this latter most important issue.
Interestingly, Davi et al. (2006) had simultaneously been studying this very possibility. Noting that "predictions for the second half of the 21st century diverge, with some models predicting that the terrestrial carbon sink will tend to level off, while others predict a decrease," they wrote that they were "hoping to shed more light on this important subject." And this they did via the use of a meteorological model and a moderate CO2 emission scenario (B2 of the IPCC) to calculate a 1960-2100 average temperature increase of 3.1°C and a mean summer rainfall decrease of 27%, which the nine scientists used as input to "a physiologically-based multi-layer process-based ecosystem productivity model (which contained a carbon allocation sub-model coupled with a soil model) to evaluate the net productivity changes of six French forest ecosystems representative of oceanic, continental and Mediterranean climates that [were] dominated, respectively, by deciduous species (Fagus sylvatica, Quercus robur), coniferous species (Pinus pinaster, Pinus sylvestris) and sclerophyllous evergreen species (Quercus ilex), which ecosystems, in their words, "are representative of a significant proportion of forests in western Europe."
"By comparing runs with and without CO2 effects," according to Davi et al., they found that "CO2 fertilization is responsible from 1960 to 2100 for an NEP [net ecosystem productivity] enhancement of about 427 g(C) on average for all sites (= 3.05 g(C) m-2 year-1)." And they also found that the CO2 fertilization effect actually turns a warming-and-drying-induced "decrease of NEP into an increase." In addition, they determined that "no saturation of this effect on NEP is found because the differences between the simulations with and without CO2 fertilization continuously increase with time." Therefore, even in the face of what was projected to be a truly "unprecedented" global warming and drying scenario, the real-world physiological effects of atmospheric CO2 enrichment that were included in the ecosystem productivity model employed by Davi et al. were able to more than compensate for the deleterious effects of the dramatic climate-change scenario on the productivity of major European forests.
About this same time, and for two full growing seasons, Sefcik et al. (2007) studied the interactive effects of elevated atmospheric CO2 concentration (658 ppm vs. the ambient concentration of 383 ppm), nitrogen (N) deposition (ambient and ambient + 30 kg N ha-1 year-1), and light availability (limited and saturated) on leaf photosynthesis, growth and survival of understory seedlings of six different hardwood tree species - paper birch (Betula papyrifera), quaking aspen (Populus tremuloides), sugar maple (Acer saccharum), American beech (Fagus grandifolia), eastern white pine (Pinus strobus) and black cherry (Prunus serotina) - which were enclosed by open-top chambers within a 90-year-old N-limited northern hardwood forest in northern Lower Michigan, USA.
Over the course of this two-year study, the 72% increase in the air's CO2 concentration increased light-limited photosynthesis in the six tree species by an average of 47%, while it increased light-saturated photosynthesis by fully 60%. With respect to survival, at low N-availability seedling survival rates were similar in the ambient and elevated CO2 treatments at 57% +/- 5% and 55% +/- 4%, respectively. In addition, as the researchers described it, "for plants grown with high N availability, those grown in ambient CO2 demonstrated 78 +/- 4% survival, while those grown in elevated CO2 exhibited the greatest survival rate of all of the treatment combinations with an 85 +/- 2% survival rate." And as a result of these multiple findings, Sefcik et al. concluded that "N deposition may alleviate some photosynthetic acclimation [i.e., down regulation] to long-term CO2 enrichment in N-limited understory seedlings." And they therefore further concluded that "increasing CO2 and nitrogen deposition from fossil fuel combustion can directly impact seedling physiology and survivorship," quite obviously for the better.
Working concurrently, Su et al. (2007) used a process-based model (BIOME-BGC) to investigate the response of Picea schrenkiana forest to future climate changes and atmospheric carbon dioxide concentration increases in the Tianshan Mountains of northwestern China, which they validated by comparing simulated net primary productivity (NPP) under current climatic conditions with independent field-measured data. The specific climate change scenario employed in this endeavor was a double-CO2-induced temperature increase of 2.6°C and a precipitation increase of 25%. And when the precipitation increase predicted by the model was considered by itself, the NPP of the P. schrenkiana forest increased by 14.5%; while the predicted temperature increase by itself increased forest NPP by 6.4%, and the CO2 increase by itself boosted NPP by 2.7%. When the predicted increases in precipitation and temperature occurred together, however, forest NPP increased by a larger 18.6%, which is just slightly less than the sum of the two individual effects. But when the CO2 concentration increase was added to the mix and all three factors increased together, the Chinese researchers found that forest NPP "increased dramatically, with an average increase of about 30.4%." So Su et al. thus concluded that "the effects of precipitation and temperature change were simply additive," but that the synergy between the effects of climate change and doubled CO2 made the whole response much larger than the sum of its separate responses, due to the fact that "feedback loops associated with the water and nitrogen cycles [which may be influenced significantly by atmospheric CO2 enrichment] ultimately influence the carbon assimilation response."
One year later, Koutavas (2008) had a study published in Dendrochronologia, wherein he wrote that "tree rings are the primary archive used in annually resolved climate reconstructions spanning recent centuries to millennia, and as such their response to non-climatic factors requires careful evaluation." Stating that an important consideration in this regard "is whether radial growth in trees over the 20th century has been influenced by anthropogenic effects, particularly the rising concentration of CO2 in the global atmosphere," he further noted that "LaMarche et al. (1984) were the first to attribute late 20th century growth enhancement in high-elevation bristlecone and limber pines from the western US to CO2 fertilization," adding that "Graybill and Idso (1993) further argued that a CO2 growth effect can be detected in tree-ring chronologies from the southwest US in species exhibiting a strip-bark morphology."
In further exploring this subject, Koutavas analyzed ring-width variations obtained from cores of eight Greek fir (Abies cephalonica) trees growing at elevations between 1300 and 1600 meters on the southern slopes of Mt. Ainos on the island of Cephalonia in the Ionian Sea west of mainland Greece, while he employed climate data from the University of East Anglia to determine whether any growth changes noted over the period of the ring-width record (AD 1840-2005) could be ascribed to any regional climate changes to which the trees might have been exposed, the results of which operations are depicted in the figure below.
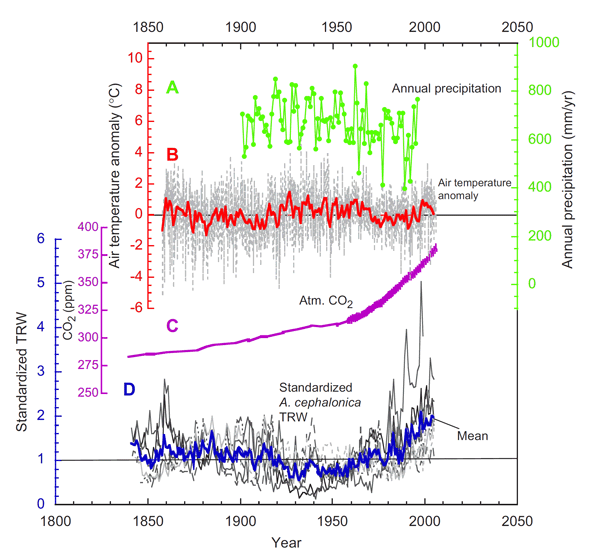
Figure 1. Annual precipitation totals, annual air temperature anomalies, atmospheric CO2 concentrations (from Mauna Loa and Antarctica's Law Dome ice core), and the mean standardized tree-ring series of the Greek fir trees. Adapted from Koutavas (2008).
As can readily be seen from these data, and as stated by Koutavas himself, there was a "strong acceleration of growth over the second half of the 20th century," and he noted that "the sustained increase in growth since 1990 in particular was unprecedented over the full length of the data set." He also correctly stated that these positive growth trends "bear no relationship to regional temperature or precipitation variations and therefore are unlikely to be climatically induced." And he affirmed that "disturbance effects from human activities are also unlikely, as the study site lies in a remote forest area with difficult access." Thus, about the only rational explanation for the late 20th-century growth acceleration seen in the ring-width data is Koutavas' suggestion that "the enhanced growth reflects a fertilization effect due to rising CO2 in the global atmosphere."
Contemporaneously, Martinez-Vilalta et al. (2008) used tree-ring data from the Catalan Ecological and Forest Inventory "to study the temporal variability of Scots pine stem radial growth (period 1901-1997) across a relatively large region (Catalonia, NE Spain) situated close to the southern limit of the distribution of the species." This inventory included a total of 10,664 plots randomly distributed throughout the forested area of Catalonia, where Scots pine was present in 30.2% of the plots, and it was the dominant tree species in 18.4% of them. In describing their results, the five researchers stated that they "showed an overall increase of 84% in Scots pine BAI [basal area increment] during the 20th century, consistent with most previous studies for temperate forests." And in this regard, they made a point of stating that "this trend was associated with increased atmospheric CO2 concentration," which they interpreted to be "a fertilization effect." There was also, however, "a marked increase in temperature across the study region (0.19°C per decade on average)," but they report that "this warming had a negative impact on radial growth, particularly at the drier sites," although "its magnitude was not enough to counteract the fertilization effect."
Moving ahead another year, Peng et al. (2009) validated the process-based TRIPLEX model of forest growth and carbon and nitrogen cycling against observed data, after which they used the calibrated model to investigate the potential impacts of projected increases in the atmosphere's CO2 concentration on the climate of northeast China and its interactions with the aerial fertilization effect of the increase in atmospheric CO2 in computing changes likely to occur in the net primary productivity (NPP) and carbon budget of the region's forests. This work revealed, first of all, that the model validation results showed that "the simulated tree total volume, NPP, total biomass and soil carbon are consistent with observed data across the Northeast of China, demonstrating that the improved TRIPLEX model is able to simulate forest growth and carbon dynamics of the boreal and temperate forest ecosystems at regional scale." Secondly, the seven scientists noted that the application of the appropriately calibrated model indicated that climate change would increase forest NPP and biomass carbon but decrease overall soil carbon under all three of the climate change scenarios they studied. However, they found that "the combined effects of climate change and CO2 fertilization on the increase of NPP were estimated to be 10-12% for [the] 2030s and 28-37% in [the] 2090s," because "the simulated effects of CO2 fertilization significantly offset the soil carbon loss due to climate change alone."
Peng et al. thus concluded that "overall, future climate change and increasing atmospheric CO2 will have a significant impact on the forest ecosystems of Northeastern China," while noting that their findings clearly indicated that that impact would be beneficial. In addition, they wrote that "the results of the effects of CO2 fertilization on NPP simulated by TRIPLEX1.0 are consistent with the recent FACE experiments in temperate forests in North America and Europe (Norby et al., 2005), global analyses of Melillo et al. (1993) and Mathews (2007), and site-specific investigations in Canadian boreal forest ecosystems (Peng and Apps, 1998, 1999)," which consistencies led them to additionally conclude that "the effect of CO2 fertilization on forest NPP is now firmly established." And it would appear that that firmly-established effect is now being widely recognized to be a firmly-established global blessing for earth's forests.
Inching another year closer to the present, Cole et al. (2010) wrote that quaking aspen (Populus tremuloides Michx.) is a dominant forest type in north-temperate, montane, and boreal regions of North America," stating that it is, in fact, "the most widely distributed tree species on the continent," while noting that aspen - and related poplars - are "quintessential foundation species (Ellison et al., 2005), shaping the structure and function of the communities and ecosystems in which they occur (Whitham et al., 2006; Schweitzer et al., 2008; Madritch et al., 2009)." This being the case, they felt it important to attempt to determine how this keystone species may have responded to the increase in atmospheric CO2 concentration that has occurred over the past several decades, especially within the context of the climatic changes that occurred concurrently.
To accomplish this goal, the four researchers collected branches from 919 trees after their leaves had dropped in the fall, obtaining samples that represented 189 genets or clones (five trees per clone) at eleven sites distributed throughout three regions of Wisconsin (USA). The sampled trees ranged from 5 to 76 years of age and came from second-growth unmanaged forests south of the areas defoliated by forest tent caterpillars in 1980-1982, 1989-1990 and 2001-2002. In addition, they recorded trunk diameter at breast height for each sampled tree, which parameter, in their words, "is very highly correlated with total biomass in aspen," citing the work of Bond-Lamberty et al. (2002).
So what did the Minnesota and Wisconsin scientists learn? First of all, they determined that "age-specific ring width increased over time," and that "the greatest increase occurred for relatively young trees, so that young trees grew faster in recent years than did young trees several decades ago." During the past half-century, for example, they found that the growth of trees 11-20 years old rose by 60%. In addition, they observed that "rising CO2 causes ring width to increase at all moisture levels, apparently resulting from improved water use efficiency," so that "the overall increase results from historical increases in both CO2 and water availability." And when they separated out the impacts of the two factors, they found that "the effect of rising CO2 had been to increase ring width by about 53%," as a result of "a 19.2% increase in ambient CO2 levels during the growing season, from 315.8 ppm in 1958 (when CO2 records began) to 376.4 ppm in 2003."
This was a truly remarkable finding; and Cole et al. commented that "the magnitude of the growth increase uncovered by this analysis raises the question of how much other major forest species may have responded to the joint effects of long-term changes in CO2 and precipitation." And in this regard, it seems logical that other tree species may well have experienced similar growth stimulations, particularly in light of the analysis of Tans (2009), who demonstrated that earth's land surfaces were a net source of CO2 to the atmosphere until about 1940 - primarily due to the felling of forests and the plowing of grasslands to make way for expanded agricultural activities - but who found that from 1940 onward, as shown in the figure below, the terrestrial biosphere had become, in the mean, an increasingly greater sink for CO2, and that it had done so even in the face of massive global deforestation, for which it apparently more than compensated. Thus, the combined findings of the two stellar studies of Tans and Cole et al. clearly testify to the phenomenal ability of the ongoing rise in the air's CO2 content to literally transform the face of the earth, as the planet's forests get a tremendous new lease on life, courtesy of mankind's mining and burning of coal, gas and oil.
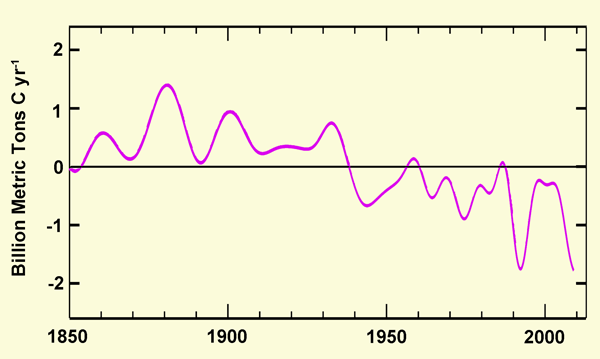
Figure 2. Five-year smoothed rates of carbon transfer from land to air (+) or from air to land (-) vs. time. Adapted from Tans (2009).
Creeping ahead yet another year, we come to the study of Knapp and Soule (2011), who wrote that "atmospheric CO2 concentrations have increased by over 27% since the early 20th century, resulting in enhanced radial tree growth in natural environments for numerous tree species in a variety of climatic regions (e.g., LaMarche et al., 1984; Knapp et al., 2001; Soule and Knapp, 2006; Voelker et al., 2006; Wang et al., 2006; Koutavas, 2008)." In addition, they also noted that "the principal benefit of elevated CO2 for radial growth has been linked to increased intrinsic water-use efficiency (iWUE), which is the ratio of net CO2 assimilation through leaf stomata to leaf stomatal conductance." And they report that "increases in iWUE based on carbon isotope chronologies have been identified for trees growing in both controlled (e.g., Leavitt et al., 2003) and natural environments (e.g., Bert et al., 1997; Feng, 1999; Tang et al., 1999; Arneth et al., 2002; Saurer et al., 2004; Waterhouse et al., 2004; Liu et al., 2007)."
In light of these well-established facts, the two researchers, as they described it, "examined radial growth responses of ponderosa pine (Pinus ponderosa var. ponderosa) between 1905-1954 and 1955-2004 to determine if the effects of increased intrinsic water-use efficiencies caused by elevated atmospheric CO2 concentrations were age-specific," working with 209 cores collected from mature trees (ranging in age from at least 100 to over 450 years) from five different sites in the USA's northern Rocky Mountains, while additionally calculating iWUE using carbon isotope data from 1850 to 2004.
This work, according to Knapp and Soule, revealed that "(1) responses to elevated atmospheric CO2 in old-growth ponderosa forests are age-specific; (2) radial growth increases in older trees coincided with increased iWUE; (3) ponderosa had increased growth rates in their third, fourth, and fifth centuries of life; and (4) age-specific growth responses during 1955-2004 are unique since at least the mid-16th century." And they also reported that "increases in iWUE during 1955-2004 were 11% greater than during 1905-1954."
In discussing their impressive findings, Knapp and Soule said they "demonstrate that old-growth ponderosa pine forests of the northern Rockies have likely benefited from the effects of increased atmospheric CO2 since the mid-20th century and that the benefits increase with tree age." And since the CO2-induced radial growth increases in the older trees "were significantly associated with rising iWUE," they opined that the "accelerated growth rates are likely caused by more efficient water use in the semiarid environment where the trees were sampled."
In concluding, the two scientists rightly stated that "old-growth trees can be highly responsive to environmental changes," especially that of the ongoing rise in the air's CO2 content, as their work clearly demonstrates to be the case. In fact, they note that even what many might call ancient trees are still "capable of increased growth rates several hundred years after establishment," citing in this regard the work of McDowell et al. (2003) and Martinez-Vilalta et al. (2007).
Dropping back a of couple years, Johnson and Abrams (2009) - using data obtained from the website of the International Tree-Ring Data Bank, as well as from cores that had been collected previously and stored in their laboratory at The Pennsylvania State University (USA) - explored growth rate (basal area increment, BAI) relationships across age classes (from young to old) for eight tree species commonly found throughout the eastern United States, namely, bigtooth aspen (Populus grandidentata Michx.), blackgum (Nyssa sylvatica Marsh.), black oak (Quercus velutina Lam.), chestnut oak (Quercus Montana L.), hemlock (Tsuga canadensis L. Carr.), pitch pine (Pinus rigida Mill.), red oak (Quercus rubra) and white oak (Quercus alba L.). And what did they learn from this exercise?
The two researchers reported that "a remarkable finding of this study was that even the oldest trees of several species had slow but increasing BAI values, which continued throughout the life of most trees." The reason they characterized this finding as "remarkable," as they went on to explain, was that it "contradicts the sigmoidal growth model that predicts growth rate should plateau and then decline, as middle age trees approach old age," citing the studies of Ryan and Yoder (1997) and Weiner and Thomas (2001). And they also reported that "over the last 50-100 years, younger trees within a species grew faster than did the older trees when they were of the same respective age," which is what Knapp and Soule (2011) also found to be the case with ponderosa pine trees in the USA's northern Rocky Mountains.
In further discussing their findings, the two researchers from Pennsylvania State University's School of Forest Resources wrote that "it seems reasonable to assume" that the greater growth rates of older trees of the current era compared to older trees of older times "may be due to a stimulatory effect of anthropogenic global change defined in the broadest sense," including "increased CO2 levels, warming temperatures, increased precipitation, and changes in precipitation chemistry," while noting that "yearly average temperatures, atmospheric CO2 and nitrogen levels have increased in the eastern US (as well as much of the rest of the world) over the last 50-100 years."
Shifting gears just a bit, Rasineni et al. (2011) introduced their study of tree growth by noting that "excess light limits photosynthesis by photo-inhibition, resulting in reduced carbon gain and also causing photo-damage (Oquist and Huner, 1993; Pastenes et al., 2003; Allakhverdiev and Murata, 2004; Nishiyama et al., 2006)," while further indicating, therefore, that "plants grown in tropical climates usually experience significantly high irradiance leading to the strong midday depression of photosynthesis (Hymus et al., 2001)."
In exploring this subject further, the three researchers made use of two open-top chambers in the Botanical Gardens of the University of Hyderabad, India, each of which contained four six-month-old specimens of the fast-growing tropical Gmelina arborea tree, which they maintained at optimum moisture and nutrient levels while they measured several plant properties and processes related to photosynthesis and photosystem II (PSII) photochemistry and photoinhibition of well-expanded and light-exposed leaves randomly chosen from the upper half of the plant canopy that they maintained at both ambient and elevated CO2 concentrations (360 and 460 ppm, respectively). This work revealed that there were no significant differences in CO2 assimilation rates between the ambient and elevated-CO2-grown plants during early morning hours; but they discovered that "photosynthesis typically maximized between 0900 hours and 1000 hours in both ambient and elevated CO2-grown plants," which thereafter experienced net photosynthetic rates of 20 and 32.5 umol/m2/s, respectively, for a stunning CO2-induced enhancement of 62%, which for a CO2 enrichment of 300 ppm would be roughly equivalent to an enhancement of 180%. Subsequently, during the midday period of 1100-1300 hours, net photosynthesis rates were still significantly enhanced by about 37% (roughly equivalent to a 300-ppm induced increase of more than 100%) in the elevated CO2 treatment, after which the difference between the two CO2 treatments once again became insignificant. And noting that the elevated CO2 treatment significantly mitigated PSII-photoinhibition "through enhanced electron transport rates and through efficient biochemical reactions in leaves of G. arborea," Rasineni et al. concluded that their data "demonstrate that future increases in atmospheric CO2 may have positive effects on photochemical efficiency in fast growing tropical tree species," allowing them to take great advantage of the high-light midday period of potential maximum growth in earth's tropical regions.
In another study from Soule and Knapp (2011), the two researchers "examined changes in and relationships between radial growth and intrinsic water-use efficiency (iWUE) of ponderosa pine (Pinus ponderosa) trees, climate, and atmospheric CO2 in the western United States since the mid-nineteenth century," developing "tree-ring chronologies for eight sites in three climate regions and using carbon isotope data to calculate pentadal values of iWUE," after which they "examined relationships among radial growth, climate, iWUE, and CO2 via correlation and regression analyses."
This work revealed, as they have described it, that "trends toward higher rates of iWUE for ponderosa pine are panregional, occurring at eight sites within three distinct climatic regimes and for two subspecies," which results, in their words, "are similar to those reported by Feng (1999) for several coniferous tree species found throughout western North America." They also noted that "increasing iWUE has been reported for conifers at other northern hemisphere locations (e.g., Bert et al., 1997; Saurer et al., 2004)," concluding that "future increases in iWUE are likely for ponderosa pine within our study regions as CO2 levels increase," and stating that they found "significant improvements in radial growth rates during drought years after 1950," which suggests that "increased iWUE associated with rising CO2 can positively impact tree growth rates in the western United States and is thus an evolving component of forest ecosystem processes."
Soule and Knapp also stated that "if potential climate changes lead to increasing aridity in the western United States, additional increases in iWUE associated with future increases in CO2 might ameliorate growth declines associated with drought conditions." Yet in spite of all of this good news, the U.S. Environmental Protection Agency and the Intergovernmental Panel on Climate Change are doing all that they possibly can to reduce anthropogenic CO2 emissions to as low as they can possibly get them to go.
Dick et al. (2012) introduced their work by noting that tropical rain forests have been "a persistent feature in South America for at least 55 million years," and stating that "at times in the past, Amazon surface air temperatures have been higher than those today (Feely and Silman, 2010; Hoorn et al., 2010; Jaramilo et al., 2010; Haywood et al., 2011)." In addition, they say "experiments show that tropical plants can photosynthesize and maintain a positive carbon balance under higher temperatures than those occurring today (Krause et al., 2010; Way and Oren, 2010)." So the question naturally arises: How high can Amazon temperatures go and its trees still survive?
In broaching this question, Dick et al. hypothesized that "the older the age of a species prior to the Pleistocene, the warmer the climate it has previously survived," noting that Pliocene and late-Miocene air temperatures of 2.6-5 million years ago (Ma) and late-Miocene air temperatures of 8-10 Ma across Amazonia were "similar to AD 2100 temperature projections under low and high carbon emission scenarios, respectively." In fact, they report that "some 56.3 Ma during the Paleocene-Eocene Thermal Maximum (PETM), global mean temperature increased by 5-6°C over a period of <= 20 ka," citing Haywood et al. (2011). And they say that "fossil pollen from the PETM showed an increase in tree diversity in three South American rainforest sites with abundant rainfall (Jaramillo et al., 2010)." Therefore, they used comparative phylogeographic analyses to determine the age of the tropical tree species that are currently found in Amazonia.
This work led to their finding that "9 of 12 widespread Amazon tree species have Pliocene or earlier lineages (>2.6 Ma), with seven dating from the Miocene (>5.6 Ma) and three >8 Ma." And as a result, they concluded, as they describe it, that "the remarkably old age of these species suggests that Amazon forests passed through warmth similar to [that predicted by climate alarmists for] AD 2100 levels and that, in the absence of other major environmental changes, near-term high temperature-induced mass species extinction is unlikely." And they therefore suggested that "direct human impacts (forest clearance, forest fragmentation, fires, and loss of seed dispersing animals), and their interactions may be more important immediate threats to the integrity of Amazon rain forests and therefore should remain a focus of conservation policy."
Closing out this topical review with a view toward the future, writing in the Canadian Journal of Forest Research, Peters et al. (2013) describe how they applied the well-tested PnET-CN ecosystem model that simulates carbon, water and nitrogen dynamics in forests over time - as per Aber et al. (1996, 1997), Ollinger et al. (2002) and Peters et al. (2012) - in order to "compare the long-term effects of changing climate and atmospheric CO2 on productivity, evapotranspiration, runoff and net nitrogen mineralization in current Great Lakes forest types," noting that outputs from this model "have been previously corroborated in the Great Lakes region for current forest productivity, net N mineralization, leaf area index, and foliar nitrogen concentrations," again citing Peters et al. (2012). This they did with the help of "two statistically downscaled climate projections, PCM B1 (warmer and wetter) and GFDL A1F1 (hotter and drier)," in order to represent "two potential future climate and atmospheric CO2 scenarios."
Results indicated that for the period 1960-2099, "changes in evapotranspiration could range from -3% to +6%, runoff could increase from 2% to 22%, and net nitrogen mineralization could increase 10% to 12%," while average regional productivity could increase from a substantial 67% to a whopping 142%! And in regard to these last two figures, they say that the increased productivity "was almost entirely driven by CO2 fertilization effects, rather than by temperature or precipitation."
In concluding this topical summary, it should be clear to everyone, based on real-world evidence garnered from a multitude of experimental endeavors, that the realization of even the most far-fetched climate-change predictions of the IPCC would be unable to stamp out the many beneficial impacts of atmospheric CO2 enrichment on the growth and development of Earth's forests, be they boreal, tropical or something in between.
References
Aber, J.D. and Driscoll, C.T. 1997. Effects of land use, climate variation, and N deposition on N cycling and C storage in northern hardwood forests. Global Biogeochemical Cycles 11: 639-648.
Aber, J.D., Reich, P.B. and Goulden, M.L. 1996. Extrapolating leaf CO2 exchange to the canopy: a generalized model of forest photosynthesis compared with measurements by eddy correlation. Oecologia 106: 257-265.
Aidar, M.P.M., Martinez, C.A., Costa, A.C., Costa, P.M.F., Dietrich, S.M.C. and Buckeridge, M.S. 2002. Effect of atmospheric CO2 enrichment on the establishment of seedlings of jatoba, Hymenaea courbaril L. (Leguminosae, Caesalpinioideae). Biota Neotropica 2: BN01602012002.
Ainsworth, E.A. and Long, S.P. 2005. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytologist 165: 351-372.
Allakhverdiev, S.I. and Murata, N. 2004. Environmental stress inhibits the synthesis de novo of proteins involved in the photodamage-repair cycle of photosystem II in Synechocystis sp. PCC 6803. Biochimica et Biophysica Acta 1657: 23-32.
Allen, O.N. and Allen, E.K. 1981. The Leguminosae. The University of Wisconsin Press, Madison, Wisconsin, USA, pp. 337-338.
Archer, S., Boutton, T.W. and Hibbard, K.A. 2001. Trees in grasslands: biogeochemical consequences of woody plant expansion. In: Schulze, E.-D., Heimann, M., Harrison, S.P., Holland, E.A., Lloyd, J., Prentice, I.C. and Schimel, D.S. (Eds.). Global Biogeochemical Cycles in the Climate System. Academic Press, San Diego, California, USA, pp. 115-137.
Archer, S., Schimel, D.S. and Holland, E.A. 1995. Mechanisms of shrubland expansion: land use, climate or CO2? Climatic Change 29: 91-99.
Arneth, A., Lloyd, J., Santruckova, H., Bird, M., Grigoryev, S., Kalaschnikov, Y.N., Sukachev, V.N., Gleixner, G. and Schulze, E.-D. 2002. Response of central Siberian Scots pine to soil water deficit and long-term trends in atmospheric CO2 concentration. Global Biogeochemical Cycles 16: 10.1029/2000GB001374.
Baker, T.R., Phillips, O.L., Malhi, Y., Almeida, S., Arroyo, L., Di Fiore, A., Erwin, T., Higuchi, N., Killeen, T.J., Laurance, S.G., Laurance, W.F., Lewis, S.L., Monteagudo, A., Neill, D.A., Núñez Vargas, P., Pitman, N.C.A., Silva, J.N.M. and Vásquez Martínez, R. 2004. Increasing biomass in Amazonian forest plots. Philosophical Transactions of the Royal Society of London Series B - Biological Sciences 359: 353-365.
Bergh, J., Freeman, M., Sigurdsson, B., Kellomaki, S., Laitinen, K., Niinisto, S., Peltola, H. and Linder, S. 2003. Modelling the short-term effects of climate change on the productivity of selected tree species in Nordic countries. Forest Ecology and Management 183: 327-340.
Bernacchi, C.J., Calfapietra, C., Davey, P.A., Wittig, V.E., Scarascia-Mugnozza, G.E., Raines, C.A. and Long, S.P. 2003. Photosynthesis and stomatal conductance responses of poplars to free-air CO2 enrichment (PopFACE) during the first growth cycle and immediately following coppice. New Phytologist 159: 609-621.
Berntson, G.M. and Bazzaz, F.A. 1998. Regenerating temperate forest mesocosms in elevated CO2: belowground growth and nitrogen cycling. Oecologia 113: 115-125.
Bert, D., Leavitt, S.W. and Dupouey, J.-L. 1997. Variations of wood ð13C and water-use efficiency of Abies alba during the last century. Ecology 78: 1588-1596.
Bond, W.J. and Midgley, G.F. 2000. A proposed CO2-controlled mechanism of woody plant invasion in grasslands and savannas. Global Change Biology 6: 865-869.
Bond, W.J., Midgley, G.F. and Woodward, F.I. 2003. The importance of low atmospheric CO2 and fire in promoting the spread of grasslands and savannas. Global Change Biology 9: 973-982.
Bond-Lamberty, B., Wang, C. and Gower, S.T. 2002. Aboveground and belowground biomass and sapwood area allometric equations for six boreal tree species of northern Manitoba. Canadian Journal of Forest Research 32: 1441-1450.
Bragg, F.J., Prentice, I.C., Harrison, S.P., Eglinton, G., Foster, P.N., Rommerskirchen, F. and Rullkotter, J. 2013. Stable isotope and modeling evidence for CO2 as a driver of glacial-interglacial vegetation shifts in southern Africa. Biogeosciences 10: 2001-2010.
Clark, D.A. 2002. Are tropical forests an important carbon sink? Reanalysis of the long-term plot data. Ecological Applications 12: 3-7.
Clark, D.A., Piper, S.C., Keeling, C.D. and Clark, D.B. 2003. Tropical rain forest tree growth and atmospheric carbon dynamics linked to interannual temperature variation during 1984-2000. Proceedings of the National Academy of Sciences, USA 100: 10.1073/pnas.0935903100.
Cole, C.T., Anderson, J.E., Lindroth, R.L. and Waller, D.M. 2010. Rising concentrations of atmospheric CO2 have increased growth in natural stands of quaking aspen (Populus tremuloides). Global Change Biology 16: 2186-2197.
Condit, R. 1997. Forest turnover, density, and CO2. Trends in Ecology and Evolution 12: 249-250.
Davi, H., Dufrene, E., Francois, C., Le Maire, G., Loustau, D., Bosc, A., Rambal, S., Granier, A. and Moors, E. 2006. Sensitivity of water and carbon fluxes to climate changes from 1960-2100 in European forest ecosystems. Agricultural and Forest Meteorology 141: 35-56.
DeLucia, E.H., Hamilton, J.G., Naidu, S.L., Thomas, R.B., Andrews, J.A., Finzi, A., Lavine, M., Matamala, R., Mohan, J.E., Hendrey, G.R. and Schlesinger, W.H. 1999. Net primary production of a forest ecosystem with experimental CO2 enrichment. Science 284: 1177-1179.
DeLucia, E.H. and Thomas, R.B. 2000. Photosynthetic responses to CO2 enrichment of four hardwood species in a forest understory. Oecologia 122: 11-19.
Dick, C.W., Lewis, S.L., Maslin, M. and Bermingham, E. 2012. Neogene origins and implied warmth tolerance of Amazon tree species. Ecology and Evolution 3: 162-169.
Duquesnay, A., Breda, N., Stievenard, M. and Dupouey, J.L. 1998. Changes of tree-ring ð13C and water-use efficiency of beech (Fagus sylvatica L.) in north-eastern France during the past century. Plant, Cell and Environment 21: 565-572.
Ehleringer, J.R., Cerling, T.E. and Helliker, B.R. C4 photosynthesis, atmospheric CO2 and climate. Oecologia 112: 285-299.
Ellison, A.M., Bank, M.S., Clinton, B.D., Colburn, E.A., Elliott, K., Ford, C.R., Foster, D.R., Kloeppel, B.D., Knoepp, J.D., Lovett, G.M., Mohan, J., Orwig, D.A., Rodenhouse, N.L., Sobczak, W.V., Stinson, K.A., Stone, J.K., Swan, C.M., Thompson, J., Holle, B.V. and Webster, J.R. 2005. Loss of foundation species: consequences for the structure and dynamics of forested ecosystems. Frontiers in Ecology and the Environment 3: 479-486.
Feeley, K.J. and Silman, M.R. 2010. Biotic attrition from tropical forests correcting for truncated temperature niches. Global Change Biology 16: 1830-1836.
Feng, X. 1999. Trends in intrinsic water-use efficiency of natural trees for the past 100-200 years: a response to atmospheric concentration. Geochimica et Cosmochimica Acta 63: 1891-1903.
Grace, J., Lloyd, J., McIntyre, J., Miranda, A.C., Meir, P., Miranda, H.S., Nobre, C., Moncrieff, J., Massheder, J., Malhi, Y., Wright, I. and Gash, J. 1995. Carbon dioxide uptake by an undisturbed tropical rain-forest in Southwest Amazonia, 1992-1993. Science 270: 778-780.
Graybill, D.A. and Idso, S.B. 1993. Detecting the aerial fertilization effect of atmospheric CO2 enrichment in tree-ring chronologies. Global Biogeochemical Cycles 7: 81-95.
Hamilton, J.G., DeLucia, E.H., George, K., Naidu, S.L., Finzi, A.C. and Schlesinger, W.H. 2002. Forest carbon balance under elevated CO2. Oecologia DOI 10.1007/s00442-002-0884-x.
Hamilton, J.G., Thomas, R.B. and DeLucia, E.H. 2001. Direct and indirect effects of elevated CO2 on leaf respiration in a forest ecosystem. Plant, Cell and Environment 24: 975-982.
Hanson, P.J., Samuelson, L.J., Wullschleger, S.D., Tabberer, T.A. and Edwards, G.S. 1994. Seasonal patterns of light-saturated photosynthesis and leaf conductance for mature and seedling Quercus rubra L. foliage: differential sensitivity to ozone. Tree Physiology 14: 1351-1366.
Hanson, P.J., Wullschleger, S.D., Norby, R.J., Tschaplinski, T.J. and Gunderson, C.A. 2005. Importance of changing CO2, temperature, precipitation, and ozone on carbon and water cycles of an upland-oak forest: incorporating experimental results into model simulations. Global Change Biology 11: 1402-1423.
Hari, P. and Arovaara, H. 1988. Detecting CO2 induced enhancement in the radial increment of trees. Evidence from the northern timberline. Scandinavian Journal of Forest Research 3: 67-74.
Hari, P., Arovaara, H., Raunemaa, T. And Hautojarvi, A. 1984. Forest growth and the effects of energy production: A method for detecting trends in the growth potential of trees. Canadian Journal of Forest Research 14: 437-440.
Haywood, A.M., Ridgwell, A., Lunt, D.J., Hill, D.J., Pound, M.J., Dowsett, H.J., Dolan, A.M., Francis, J.E., and Williams, M. 2011. Are there pre-Quaternary geological analogues for a future greenhouse warming? Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences 369: 933-956.
Herrick, J.D. and Thomas, R.B. 1999. Effects of CO2 enrichment on the photosynthetic light response of sun and shade leaves of canopy sweetgum trees (Liquidambar styraciflua) in a forest ecosystem. Tree Physiology 19: 779-786.
Hoorn, C., Wesselingh, F.P., ter Steege, H., Bermudez, M.A., Mora, A., Sevink, J., Sanmartin, I., Sanchez-Meseguer, A., Anderson, C.L., Figueiredo, J.P., Jaramillo, C., Riff, D., Negri, F.R., Hooghiemstra, H., Lundberg, J., Stadler, T., Sarkinen, T. and Antonelli, A. 2010. Amazonia through time : Andean uplift, climate change, landscape evolution, and biodiversity. Science 330: 927-931.
Houghton, J.T., Ding, Y., Griggs, D.J., Noguer, M., van der Linden, P.J., Dai, X., Maskell, K. and Johnson, C.A. 2001. Climate Change 2001. In: Maskell, K. and Johnson, C.A. (Eds.). The Scientific Basis: Contribution of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change (IPCC). Cambridge University Press, Cambridge, United Kingdom.
Hussain, M., Kubiske, M.E. and Connor, K.F. 2001. Germination of CO2-enriched Pinus taeda L. seeds and subsequent seedling growth responses to CO2 enrichment. Functional Ecology 15: 344-350.
Hymus, G.J., Baker, N.R. and Long, S.P. 2001. Growth in elevated CO2 can both increase and decrease photochemistry and photoinhibition of photosynthesis in a predictable manner. Dactylis glomerata growth in two levels of nitrogen nutrition. Plant Physiology 127: 1204-1211.
Hymus, G.J., Ellsworth, D.S., Baker, N.R. and Long, S.P. 1999. Does free-air carbon dioxide enrichment affect photochemical energy use by evergreen trees in different seasons? A chlorophyll fluorescence study of mature loblolly pine. Plant Physiology 120: 1183-1191.
Idso, S.B. 1991a. The aerial fertilization effect of CO2 and its implications for global carbon cycling and maximum greenhouse warming. Bulletin of the American Meteorological Society 72: 962-965.
Idso, S.B. 1991b. Reply to comments of L.D. Danny Harvey, Bert Bolin, and P. Lehmann. Bulletin of the American Meteorological Society 72: 1910-1914.
Idso, S.B. 1995. CO2 and the Biosphere: The Incredible Legacy of the Industrial Revolution. Department of Soil, Water and Climate, University of Minnesota, St. Paul, Minnesota, USA.
Jaramillo, C., Ochoa, D., Contreras, L., Pagani, M., Carvajal-Ortiz, H., Pratt, L.M., Krishnan, S., Cardona, A., Romero, M., Quiroz, L., Rodriguez, G., Rueda, M.J., Parra, F., Moron, S., Green, W., Bayona, G., Montes, C., Quintero, O., Ramirez, R., Mora, G., Schouten, S., Bermudez H., Navarrete, R., Parra, F., Alvarán, M., Osorno, J., Crowley, J., Valencia, V. and Vervoort, J. 2010. Effects of rapid global warming at the Paleocene-Eocene boundary on neotropical vegetation. Science 330: 957-961.
Johnson, S.E. and Abrams, M.D. 2009. Age class, longevity and growth rate relationships: protracted growth increases in old trees in the eastern United States. Tree Physiology 29: 1317-1328.
Kgope, B.S., Bond, W.J. and Midgley, G.F. 2010. Growth responses of African savanna trees implicate atmospheric [CO2] as a driver of past and current changes in savanna tree cover. Austral Ecology 35: 451-463.
Knapp, P.A. and Soule, P.T. 2011. Increasing water-use efficiency and age-specific growth responses of old-growth ponderosa pine trees in the Northern Rockies. Global Change Biology 17: 631-641.
Knapp, P.A., Soule, P.T. and Grissino-Mayer, H.D. 2001. Detecting the potential regional effects of increased atmospheric CO2 on growth rates of western juniper. Global Change Biology 7: 903-917.
Knight, C.L., Briggs, J.M. and Nellis, M.D. 1994. Expansion of gallery forest on Konza Prairie Research Natural Area, Kansas, USA. Landscape Ecology 9: 117-125.
Koutavas, A. 2008. Late 20th century growth acceleration in Greek firs (Aibes cephalonica) from Cephalonia Island, Greece: A CO2 fertilization effect? Dendrochronologia 26: 13-19.
Krause, G.H., Winter, K., Krause, B., Jahns, P., Garcia, M., Aranda, J. and Virgo, A. 2010. High-temperature tolerance of a tropical tree, Ficus insipida: methodological reassessment and climate change considerations. Functional Plant Biology 37: 890-900.
LaMarche Jr., V.C., Graybill, D.A., Fritts, H.C. and Rose, M.R. 1984. Increasing atmospheric carbon dioxide: Tree ring evidence for growth enhancement in natural vegetation. Science 223: 1019-1021.
Laurance, W.F., Nascimento, H.E.M., Laurance, S.G., Condit, R., D'Angelo, S. and Andrade, A. 2004b. Inferred longevity of Amazonian rainforest trees based on a long-term demographic study. Forest Ecology and Management 190: 131-143.
Laurance, W.F., Oliveira, A.A., Laurance, S.G., Condit, R., Dick, C.W., Andrade, A., Nascimento, H.E.M., Lovejoy, T.E. and Ribeiro, J.E.L.S. 2005. Altered tree communities in undisturbed Amazonian forests: A consequence of global change? Biotropica 37: 160-162.
Laurance, W.F., Oliveira, A.A., Laurance, S.G., Condit, R., Nascimento, H.E.M., Sanchez-Thorin, A.C., Lovejoy, T.E., Andrade, A., D'Angelo, S. and Dick, C. 2004a. Pervasive alteration of tree communities in undisturbed Amazonian forests. Nature 428: 171-175.
Leavitt, S.W., Idso, S.B., Kimball, B.A., Burns, J.M., Sinha, A. and Stott, L. 2003. The effect of long-term atmospheric CO2 enrichment on the intrinsic water-use efficiency of sour orange trees. Chemosphere 50: 217-222.
Lewis, S.L. 2006. Tropical forests and the changing earth system. Philosophical Transactions of the Royal Society B 361: 195-210.
Lewis, S.L., Malhi, Y. and Phillips, O.L. 2004b. Fingerprinting the impacts of global change on tropical forests. Philosophical Transactions of the Royal Society of London Series B - Biological Sciences 359: 437-462.
Lewis, S.L., Phillips, O.L., Baker, T.R., Lloyd, J., Malhi, Y., Almeida, S., Higuchi, N., Laurance, W.F., Neill, D.A., Silva, J.N.M., Terborgh, J., Lezama, A.T., Vásquez Martinez, R., Brown, S., Chave, J., Kuebler, C., Núñez Vargas, P. and Vinceti, B. 2004a. Concerted changes in tropical forest structure and dynamics: evidence from 50 South American long-term plots. Philosophical Transactions of the Royal Society of London Series B - Biological Sciences 359: 421-436.
Lewis, S.L., Phillips, O.L., Sheil, D., Vinceti, B., Baker, T.R., Brown, S., Graham, A.W., Higuchi, N., Hilbert, D.W., Laurance, W.F., Lejoly, J., Malhi, Y., Monteagudo, A., Vargas, P.N., Sonke, B., Nur Supardi, M.N., Terborgh, J.W. and Vasquez, M.R. 2005. Tropical forest tree mortality, recruitment and turnover rates: calculation, interpretation and comparison when census intervals vary. Journal of Ecology 92: 929-944.
Liu, X., Shao, X., Liang, E., Zhao, L., Chen, T., Qin, D. and Ren, J. 2007. Species dependent responses of juniper and spruce to increasing CO2 concentration and to climate in semi-arid and arid areas of northwestern China. Plant Ecology 193: 195-209.
Lloyd, J. 1999. The CO2 dependence of photosynthesis, plant growth responses to elevated CO2 concentrations and their interaction with soil nutrient status, II. Temperate and boreal forest productivity and the combined effects of increasing CO2 concentrations and increased nitrogen deposition at a global scale. Functional Ecology 13: 439-459.
Long, S.P. 1991. Modification of the response of photosynthetic productivity to rising temperature by atmospheric CO2 concentrations: Has its importance been underestimated? Plant, Cell and Environment 14: 729-739.
Macinnis-Ng, C., Zeppel, M., Williams, M. and Eamus, D. 2011. Applying a SPA model to examine the impact of climate change on GPP of open woodlands and the potential for woody thickening. Ecohydrology 4: 379-393.
Madritch, M.D., Greene, S.G. and Lindroth, R.L. 2009. Genetic mosaics of ecosystem functioning across aspen-dominated landscapes. Oecologia 160: 119-127.
Malhi Y. and Grace, J. 2000. Tropical forests and atmospheric carbon dioxide. Trends in Ecology and Evolution 15: 332-337.
Malhi, Y. Nobre, A.D., Grace, J., Kruijt, B., Pereira, M.G.P., Culf, A. and Scott, S. 1998. Carbon dioxide transfer over a Central Amazonian rain forest. Journal of Geophysical Research 103: 31,593-31,612.
Martinez-Vilalta, J., Lopez, B.C., Adell, N., Badiella, L. and Ninyerola, M. 2008. Twentieth century increase of Scots pine radial growth in NE Spain shows strong climate interactions. Global Change Biology 14: 2868-2881.
Martinez-Vilalta, J., Vanderklein, D. and Mencuccini, M. 2007. Tree height and age-related decline in growth in Scots pine (Pinus sylvestris L.). Oecologia 150: 529-544.
Matthews, H.D. 2007. Implications of CO2 fertilization for future climate change in a coupled climate-carbon model. Global Change Biology 13: 1-11.
McDowell, N., Brooks, J.R., Fitzgerald, S.A. and Bond, B.J. 2003. Carbon isotope discrimination and growth response of old Pinus ponderosa trees to stand density reductions. Plant, Cell and Environment 26: 631-644.
Medlyn, B.E., Badeck. F.-W., De Pury, D.G.G., Barton, C.V.M., Broadmeadow, M., Ceulemans, R., De Angelis, P., Forstreuter, M., Jach, M.E., Kellomaki, S., Laitat, E., Marek, M., Philippot, S., Rey, A., Strassemeyer, J., Laitinen, K., Liozon, R., Portier, B., Roberntz, P., Wang, K. and Jarvis, P.G. 1999. Effects of elevated [CO2] on photosynthesis in European forest species: a meta-analysis of model parameters. Plant, Cell and Environment 22: 1475-1495.
Melillo, J.M., McGuire, A.D., Kicklighter, D.W., Moore, B., Vorosmarty, C.J. and Schloss, A.L. 1993. Global climate change and terrestrial net primary production. Nature 363: 234-240.
Miglietta, F., Peressotti, A., Vaccari, F.P., Zaldei, A., deAngelis, P. and Scarascia-Mugnozza, G. 2001. Free-air CO2 enrichment (FACE) of a poplar plantation: The POPFACE fumigation system. New Phytologist 150: 465-476.
Naidu, S.L. and DeLucia, E.H. 1999. First-year growth response of trees in an intact forest exposed to elevated CO2. Global Change Biology 5: 609-613.
National Assessment Synthesis Team (NAST). 2000. Climate Change Impacts on the United States: The Potential Consequences of Climate Variability and Change. US Global Change Research Program, Washington, DC.
Naumburg, E. and Ellsworth, D.S. 2000. Photosynthetic sunfleck utilization potential of understory saplings growing under elevated CO2 in FACE. Oecologia 122: 163-174.
Nelson, B.W. 2005. Pervasive alteration of tree communities in undisturbed Amazonian forests. Biotropica 37: 158-159.
Nishiyama, Y., Allakhverdiev, S.I. and Murata, N. 2006. A new paradigm for the action of reactive oxygen species in the photoinhibition of photosystem II. Biochimica et Biophysica Acta 1757: 742-749.
Norby, R.J., DeLucia, E.H., Gielen, B., Calfapietra, C., Giardina, C.P., King, S.J., Ledford, J., McCarthy, H.R., Moore, D.J.P., Ceulemans, R., De Angelis, P., Finzi, A.C., Karnosky, D.F., Kubiske, M.E., Lukac, M., Pregitzer, K.S., Scarasci-Mugnozza, G.E., Schlesinger, W.H. and Oren, R. 2005. Forest response to elevated CO2 is conserved across a broad range of productivity. Proceedings of the National Academy of Sciences USA 102: 18,052-18,056.
Norby, R.J., Hanson, P.J., O'Neill, E.G., Tschaplinski, T.J., Weltzin, J.F., Hansen, R.A., Cheng, W., Wullschleger, S.D., Gunderson, C.A., Edwards, N.T. and Johnson, D.W. 2002. Net primary productivity of a CO2-enriched deciduous forest and the implications for carbon storage. Ecological Applications 12: 1261-1266.
Norby, R.J., Todd, D.E., Fults, J. and Johnson, D.W. 2001. Allometric determination of tree growth in a CO2-enriched sweetgum stand. New Phytologist 150: 477-487.
Ollinger, S.V., Aber, J.D., Reich, P.B. and Freuder, R.J. 2002. Interactive effects of nitrogen deposition, tropospheric ozone, elevated CO2 and land use history on the carbon dynamics of northern hardwood forests. Global Change Biology 8: 545-562.
Olsson, E.G.A., Austrheim, G. and Grenne, S.N. 2000. Landscape change patterns in mountains, land use and environmental diversity, Mid-Norway 1960-1993. Landscape Ecology 15: 155-170.
Oquist, G. and Huner, N.P.A. 1993. Cold-hardening-induced resistance to photoinhibition of photosynthesis in winter rye is dependent upon an increased capacity for photosynthesis. Planta 189: 150-156.
Parker, M.L. 1987. Recent abnormal increase in tree-ring widths: A possible effect of elevated atmospheric carbon dioxide. In: Jacoby Jr., G.C. and Hornbeck, J.W. (Eds.), Proceedings of the International Symposium on Ecological Aspects of Tree-Ring Analysis. U.S. Department of Energy, Washington, DC, pp. 511-521.
Pastenes, C., Santa-Maria, E., Infante, R. and Franck, N. 2003. Domestication of the Chilean guava (Ugni molinae Turcz.) a forest understory shrub, must consider light intensity. Scientia Horticulturae 98: 71-84.
Peng, C.H. and Apps, M.J. 1998. Simulating carbon dynamics along the Boreal Forest Transect Case Study (BFTCS) in the Central of Canada: II. Sensitivity to climate change. Global Biogeochemical Cycles 12: 393-402.
Peng, C.H. and Apps, M.J. 1999. Modeling response of net primary productivity (NPP) of boreal forest ecosystems to changes in climate and fire disturbance regimes. Ecological Modeling 122: 175-193.
Peng, C., Zhou, X., Zhao, S., Wang, X., Zhu, B., Piao, S. and Fang, J. 2009. Quantifying the response of forest carbon balance to future climate change in Northeastern China: Model validation and prediction. Global and Planetary Change 66: 179-194.
Peters, E.B., Wythers, K.R., Bradford, J.B. and Reich, P.B. 2012. Influence of disturbance on temperate forest productivity. Ecosystems 16: 95-110.
Peters, E.B., Wythers, K.R., Zhang, S., Bradford, J.B. and Reich, P.B. 2013. Potential climate change impacts on temperate forest ecosystem processes. Canadian Journal of Forest Research 43: 939-950.
Phillips, O.L., Baker, T.R., Arroyo, L., Higuchi, N., Killeen, T.J., Laurance, W.F., Lewis, S.L., Lloyd, J., Malhi, Y., Monteagudo, A., Neill, D.A., Núñez Vargas, P., Silva, J.N.M., Terborgh, J., Vásquez Martínez, R., Alexiades, M., Almeida, S., Brown, S., Chave, J., Comiskey, J.A., Czimczik, C.I., Di Fiore, A., Erwin, T., Kuebler, C., Laurance, S.G., Nascimento, H.E.M., Olivier, J., Palacios, W., Patiño, S., Pitman, N.C.A., Quesada, C.A., Saldias, M., Torres Lezama, A., B. and Vinceti, B. 2004. Pattern and process in Amazon tree turnover: 1976-2001. Philosophical Transactions of the Royal Society of London Series B - Biological Sciences 359: 381-407.
Phillips, O.L. and Gentry, A.H. 1994. Increasing turnover through time in tropical forests. Science 263: 954-958.
Phillips, O.L., Malhi, Y., Higuchi, N., Laurance, W.F., Nunez, P.V., Vasquez, R.M., Laurance, S.G., Ferreira, L.V., Stern, M., Brown, S. and Grace, J. 1998. Changes in the carbon balance of tropical forests: Evidence from long-term plots. Science 282: 439-442.
Phillips, O.L., Malhi, Y., Vinceti, B., Baker, T., Lewis, S.L., Higuchi, N., Laurance, W.F., Vargas, P.N., Martinez, R.V., Laurance, S., Ferreira, L.V., Stern, M., Brown, S. and Grace, J. 2002. Changes in growth of tropical forests: Evaluating potential biases. Ecological Applications 12: 576-587.
Pimm, S.L. and Sugden, A.M. 1994. Tropical diversity and global change. Science 263: 933-934.
Prentice, I.C., Farquhar, G.D., Fasham, M.J.R., Goulden, M.L., Heimann, M., Jaramillo, V.J., Kheshgi, H.S., Le Quere, C., Scholes, R.J., Wallace, D.W.R., Archer, D., Ashmore, M.R., Aumont, O., Baker, D., Battle, M., Bender, M., Bopp, L.P., Bousquet, P., Caldeira, K., Ciais, P., Cox, P.M., Cramer, W., Dentener, F., Enting, I.G., Field, C.B., Friedlingstein, P., Holland, E.A., Houghton, R.A., House, J.I., Ishida, A., Jain, A.K., Janssens, I.A., Joos, F., Kaminski, T., Keeling, C.D., Keeling, R.F., Kicklighter, D.W., Hohfeld, K.E., Knorr, W., Law, R., Lenton, T., Lindsay, K., Maier-Reimer, E., Manning, A.C., Matear, R.J., McGuire, A.D., Melillo, J.M., Meyer, R., Mund, M., Orr, J.C., Piper, S., Plattner, K., Rayner, P.J., Sitch, S., Slater, R., Taguchi, S., Tans, P.P., Tian, H.Q., Weirig, M.F., Whorf, T. and Yool, A. 2001. The carbon cycle and atmospheric carbon dioxide. Chapter 3 of the Third Assessment Report of the Intergovernmental Panel on Climate Change. Climate Change 2001: The Scientific Basis. Cambridge University Press, Cambridge, UK, pp. 183-238.
Pritchard, S.G., Davis, M.A., Mitchell, R.J., Prior, A.S., Boykin, D.L., Rogers, H.H. and Runion, G.B. 2001. Root dynamics in an artificially constructed regenerating longleaf pine ecosystem are affected by atmospheric CO2 enrichment. Environmental and Experimental Botany 46: 35-69.
Rasineni, G.K., Guha, A. and Reddy, A.R. 2011. Elevated atmospheric CO2 mitigated photoinhibition in a tropical tree species, Gmelina arborea. Journal of Photochemistry and Photobiology B: Biology 103: 159-165.
Rathgeber, C., Nicault, A., Guiot, J., Keller, T., Guibal, F. and Roche, P. 2000. Simulated responses of Pinus halepensis forest productivity to climatic change and CO2 increase using a statistical model. Global and Planetary Change 26: 405-421.
Rathgeber, C., Nicault, A., Kaplan, J.O. and Guiot, J. 2003. Using a biogeochemistry model in simulating forests productivity responses to climatic change and [CO2] increase: example of Pinus halepensis in Provence (south-east France). Ecological Modelling 166: 239-255.
Rodrigues, R.R. and Nave, A.G. 2000. Heterogeneidade floristica das matas ciliares. In: Rodrigues, R.R. and Leitao Filho, H.F. (Eds.) Matas Ciliares: Conservacao e Recuperacao. Editora da USP/FAPESP, 2000. Sao Paulo, Brazil, pp. 45-71.
Ryan, M.G. and Yoder, B.J. 1997. Hydraulic limits to tree height and tree growth. Bioscience 47: 235-242.
Saurer, M.S., Siegwolf, R.T.W. and Schweingruber, F. 2004. Carbon isotope discrimination indicates improving water-use efficiency of trees in northern Eurasia over the last 100 years. Global Change Biology 10: 2109-2120.
Schweitzer, J.A., Madritch, M.D., Bailey, J.K., LeRoy, C.J., Fischer, D.G., Rehill, B.J., Lindroth, R.L., Hagerman, A.E., Wooley, S.C., Hart, S.C. and Whitham, T.G. 2008. The genetic basis of condensed tannins and their role in nutrient regulation in a Populus model system. Ecosystems 11: 1005-1020.
Sefcik, L.T., Zak, D.R. and Ellsworth, D.S. 2007. Seedling survival in a northern temperate forest understory is increased by elevated atmospheric carbon dioxide and atmospheric nitrogen deposition. Global Change Biology 13: 132-146.
Shaw, M.R., Zavaleta, E.S., Chiariello, N.R., Cleland, E.E., Mooney, H.A. and Field, C.B. 2002. Grassland responses to global environmental changes suppressed by elevated CO2. Science 298: 1987-1990.
Sheil, D. 1995. Evaluating turnover in tropical forests. Science 268: 894.
Sheil, D. and May, R.M. 1996. Mortality and recruitment rate evaluations in heterogeneous tropical forests. Journal of Ecology 84: 91-100.
Soule, P.T. and Knapp, P.A. 2006. Radial growth rate increases in naturally-occurring ponderosa pine trees: a late 20th century CO2 fertilization effect? New Phytologist 171: 379-390.
Soule, P.T. and Knapp, P.A. 2011. Radial growth and increased water-use efficiency for ponderosa pine trees in three regions in the western United States. The Professional Geographer 63: 379-391.
Street-Perrott, F.A., Huang, Y., Perrott, R.A., Eglinton, G., Barker, P., Khelifa, L.B., Harkness, D.D. and Olago, D.O. 1997. Impact of lower atmospheric carbon dioxide on tropical mountain ecosystems. Science 278: 1422-1426.
Su, H.-X. and Sang, W.-G. 2004. Simulations and analysis of net primary productivity in Quercus liaotungensis forest of Donglingshan Mountain Range in response to different climate change scenarios. Acta Botanica Sinica 46: 1281-1291.
Su, H.-X., Sang, W., Wang, Y. and Ma, K. 2007. Simulating Picea schrenkiana forest productivity under climatic changes and atmospheric CO2 increase in Tianshan Mountains, Xinjiang Autonomous Region, China. Forest Ecology and Management 246: 273-284.
Tang, K., Feng, X. and Funkhouser, G. 1999. The ð13C of trees in full-bark and strip-bark bristlecone pines in the White Mountains of California. Global Change Biology 5: 33-40.
Tans, P. 2009. An accounting of the observed increase in oceanic and atmospheric CO2 and an outlook for the future. Oceanography 22: 26-35.
Voelker, S.L., Muzika, R., Guyette, R.P. and Stambaugh, M.C. 2006. Evidence for historic CO2 enhancement of tree-ring growth shows a decline through age in Quercus velutina, Quercus coccinea and Pinus echinata. Ecological Monographs 76: 549-564.
Walker, R.F., Johnson, D.W., Geisinger, D.R. and Ball, J.T. 2000. Growth, nutrition, and water relations of ponderosa pine in a field soil as influenced by long-term exposure to elevated atmospheric CO2. Forest Ecology and Management 137: 1-11.
Wang, G.G., Chhin, S. and Baurle, W.L. 2006. Effect of natural atmospheric CO2 fertilization suggested by open-grown white spruce in a dry environment. Global Change Biology 12: 601-610.
Waterhouse, J.S., Switsur, V.R., Barker, A.C., Carter, A.H.C., Hemming, D.L., Loader, N.J. and Robertson, I. 2004. Northern European trees show a progressively diminishing response to increasing atmospheric carbon dioxide concentrations. Quaternary Science Reviews 23: 803-810.
Way, D.A. and Oren, R. 2010. Differential responses to changes in growth temperature between trees from different functional groups and biomes: a review and synthesis of data. Tree Physiology 30: 669-688.
Weaver, P.L. and Murphy, P.G. 1990. Forest structure and productivity in Puerto Rico's Luquillo Mountains. Biotropica 22: 69-82.
Weiner, J. and Thomas, S.C. 2001. The nature of tree growth and the age-related decline in forest productivity. Oikos 94: 374-376.
West, D.C. 1988. Detection of forest response to increased atmospheric carbon dioxide. In: Koomanoff, F.A. (Ed.), Carbon Dioxide and Climate: Summaries of Research in FY 1988. U.S. Department of Energy, Washington, D.C., p. 57.
West, D.C., Doyle, T.W., Tharp, M.L., Beauchamp, J.J., Platt, W.J. and Downing, D.J. 1993. Recent growth increases in old-growth longleaf pine. Canadian Journal of Forest Research 23: 846-853.
Whitham, T.G., Bailey, J.K. and Schweitzer, J.A. 2006. A framework for community and ecosystem genetics from genes to ecosystems. Nature Reviews Genetics 7: 510-523.
Winnett, S.M. 1998. Potential effects of climate change on US forests: a review. Climate Research 11: 39-49.
Wullschleger, S.D. and Norby, R.J. 2001. Sap velocity and canopy transpiration in a sweetgum stand exposed to free-air CO2 enrichment (FACE). New Phytologist 150: 489-498.
Last updated 16 December 2014



