The State of Earth's Terrestrial Biosphere:
How is it Responding to Rising Atmospheric CO2 and Warmer Temperatures?
Continental-Scale Analyses of Terrestrial Productivity: South America
Focusing on vegetative trends in South America, we begin with the long view of the situation provided by Beerling and Mayle (2006), who investigated the nature of Amazonian ecosystem response to the large-scale environmental changes experienced during glacial-interglacial cycles via a series of 21,000-year simulations. This they accomplished using a dynamic process-based ecosystem model for three scenarios: (1) real-world glacial-to-interglacial changes in CO2 concentration and climate, (2) the real-world change in CO2 with a constant preindustrial climate, and (3) the real-world change in climate with a constant preindustrial CO2 concentration. So what did they learn?
During the last glacial maximum, according to the authors, the model suggested that "total above-ground carbon storage in Amazonia was half preindustrial values, indicative of rain forests with markedly lower canopy densities and simpler structures due to lowered CO2 levels, corroborating modeling studies by Cowling (2004) and Cowling et al. (2001)." Thereafter, they say that "biome shifts in ecotonal areas since the last glacial maximum ["the competitive replacement of drought-adapted vegetation (e.g. savanna or deciduous/semideciduous dry forest) by rain forest"] were driven predominantly by climate change, while coincident, increased ecosystem carbon storage throughout the Amazon Basin was driven largely by CO2."
What do these findings have to do with today? In answering this question, Beerling and Mayle say "the underlying cause for the observed trend of increasing biomass in long-term Amazonian forest plots over recent years, despite drought-induced El Niño events (Phillips et al., 1998; Baker et al., 2004a), has been a subject of considerable debate (Baker et al., 2004a; Wright, 2005)," and in this regard they conclude that "this biomass increase is part of a long-term historical trend driven by anthropogenically induced rising CO2 levels since the 19th century."
In another model-based study, but covering only the late 20th century, Ichii et al. (2005) "simulated and analyzed 1982-1999 Amazonian, African, and Asian carbon fluxes using the Biome-BGC prognostic carbon cycle model driven by National Centers for Environmental Prediction reanalysis daily climate data," after which they "calculated trends in gross primary productivity (GPP) and net primary productivity (NPP)." In doing so, they found solar radiation variability to be the primary factor responsible for interannual variations in GPP, followed by temperature and precipitation variability. In terms of GPP trends, however, they report that "recent changes in atmospheric CO2 and climate promoted terrestrial GPP increases with a significant linear trend in all three tropical regions." In the Amazonian region, for which we are most interested in terms of the present discussion, the rate of GPP increase was the highest at 0.67 PgC year-1 per decade, while in Africa and Asia it was about 0.3 PgC year-1 per decade. Likewise, as with Beerling and Mayle, carbon dioxide was determined to be the major cause of increased growth, as Ichii et al. report that "CO2 fertilization effects strongly increased recent NPP trends in regional totals."
Such a finding, to say the least, is especially interesting, because for most of the past century it was believed that old-growth forests, such as those of Amazonia, should be close to dynamic equilibrium. Just the opposite, however, has been repeatedly observed by several different groups of researchers over the past two decades.
In one of the first studies to illuminate this reality, Phillips and Gentry (1994) analyzed the turnover rates - which are close correlates of net productivity (Weaver and Murphy, 1990) - of forty tropical forests from all around the world. And in doing so, they found that the growth rates of these already highly productive forests have been rising ever higher since at least 1960, and that they have experienced an apparent acceleration in growth rate sometime after 1980. Commenting on these findings, Pimm and Sugden (1994) reported that the consistency and simultaneity of the forest growth trends that Phillips and Gentry had documented on several continents led them to conclude that "enhanced productivity induced by increased CO2 is the most plausible candidate for the cause of the increased turnover."
A few years later, Phillips et al. (1998) analyzed forest growth rate data for the period 1958 to 1996 for several hundred plots of mature tropical trees scattered around the world, finding that tropical forest biomass, as a whole, increased substantially over the period of record (Figure 26). In fact, the increase in the Neotropics (tropical Central and South America) was equivalent to approximately 40% of the missing terrestrial carbon sink of the entire globe. And, again, they identified the aerial fertilization effect of the ongoing rise in the air's CO2 content as one of the primary factors likely to be responsible for this phenomenon.
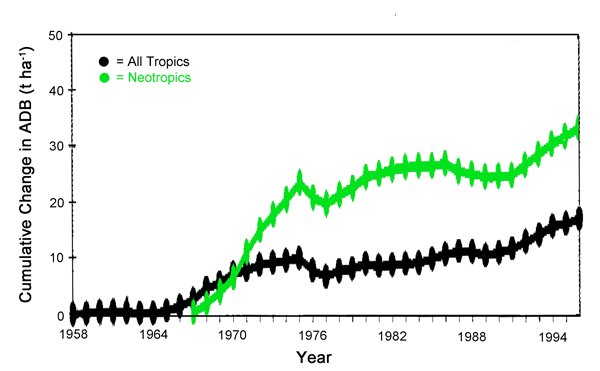
Figure 26. Cumulative aboveground dry biomass change (ADB) per year in humid forests in the entire Tropics since 1958 and in the Neotropics (tropical Central and South America) since 1967. Adapted from Phillips et al. (1998).
More recently, Laurance et al. (2004a) reported accelerated growth in the 1990s relative to the 1980s for the large majority (87%) of tree genera in 18 one-hectare plots spanning an area of about 300 km2 in central Amazonia, while Laurance et al. (2004b) observed similarly accelerated tree community dynamics in the 1990s relative to the 1980s. And once again, it was suggested, in the words of Laurance et al. (2005), that these "pervasive changes in central Amazonian tree communities were most likely caused by global- or regional-scale drivers, such as increasing atmospheric CO2 concentrations (Laurance et al., 2004a,b)."
Expanding upon this theme, Laurance et al. (2005) say they "interpreted these changes as being consistent with an ecological 'signature' expected from increasing forest productivity (cf., Phillips and Gentry, 1994; Lewis et al. 2004a,b; Phillips et al., 2004)." They note, however, that they have been challenged in this conclusion by Nelson (2005), and they thus go on to consider his arguments in some detail, methodically dismantling them one by one.
Next on the scene was Lewis (2006), who reports that the increasing dynamism and productivity of intact tropical forests has had a long history, noting that "across the paleotropics forest dynamism has been steadily increasing for five decades (Phillips and Gentry, 1994)." Among 50 old-growth plots scattered across tropical South America, for example, he notes that "stem recruitment, stem mortality, and biomass growth, and loss, all increased significantly (Lewis et al., 2004a)." In addition, he reports that "over approximately the last 20 years, long-term monitoring of 59 plots showed that above-ground biomass increased by 0.6 ± 0.2 tonnes C ha-1 a-1, or a relative increase of 0.50 ± 0.17% a-1 (mean ± 95% confidence interval; Baker et al., 2004a)." This rate of increase, to quote him again, "is slightly higher than that documented by Phillips et al. (1998)." Thus, there is no question that "over the past two decades," according to Lewis, "these forests have shown concerted changes in their ecology, becoming, on average, faster growing - more productive - and more dynamic, and showing a net increase in above-ground biomass," all of which rates of increase are greater than the previously documented increases in the rates of these phenomena.
As for what might be causing this suite of changes, Lewis states that "the results appear to show a coherent fingerprint of increasing net primary productivity across tropical South America, caused by a long-term increase in resource availability (Lewis et al., 2004a,b)." And as for what "resources" might be involved, Lewis gives four possibilities: increases in solar radiation, air temperature, nutrient deposition and atmospheric CO2 concentration. But after analyzing each of them in detail, he concludes that "the most parsimonious explanation is the increase in atmospheric CO2, because of the undisputed long-term historical increase in CO2 concentrations, the key role of CO2 in photosynthesis, and the demonstrated positive effects of CO2 fertilization on plant growth rates including experiments on whole temperate-forest stands (Ainsworth and Long, 2005)," or as he states in another place in his review, the explanation resides in "the anthropogenic increase in atmospheric carbon dioxide concentrations, increasing forest net primary productivity leading to accelerated forest growth and dynamics."
In spite of the forest growth optimism inherent in the studies cited above, some pessimists remained. As background for discussing their reservations, Gloor et al. (2009) wrote that "analysis of earlier tropical plot data has suggested that large-scale changes in forest dynamics are currently occurring in Amazonia (Phillips and Gentry, 1994; Phillips et al., 2004a), and that an increase in aboveground biomass has occurred, with increases in mortality tending to lag increases in growth (Phillips et al., 1998; Baker et al., 2004a,b; Lewis et al., 2004a)." And they state that this conclusion had been challenged by an overzealous application of the "Slow in, Rapid out" dictum, which relates to the fact that forest growth is a slow process, whereas mortality can be dramatic and singular in time, such that sampling over relatively short observation periods may miss these more severe events, leading to positively-biased estimates of aboveground biomass trends, when either no trend or negative trends actually exist.
In an attempt to resolve this debate, Gloor et al. statistically characterized the disturbance process in Amazon old-growth forests as recorded in 135 forest plots of the RAINFOR network up to 2006, as well as other independent research programs, exploring the consequences of sampling artifacts using a data-based stochastic simulator. Their results indicated that "over the observed range of annual aboveground biomass losses, standard statistical tests show that the distribution of biomass losses through mortality follow an exponential or near-identical Weibull probability distribution and not a power law as assumed by others." In addition, they say that "the simulator was parameterized using both an exponential disturbance probability distribution as well as a mixed exponential-power law distribution to account for potential large-scale blow-down events," and that "in both cases, sampling biases turn out to be too small to explain the gains detected by the extended RAINFOR plot network." And based on these findings, Gloor et al. concluded that their results lend "further support to the notion that currently observed biomass gains for intact forests across the Amazon are actually occurring over large scales at the current time, presumably as a response to climate change," which in many of their earlier papers is explicitly stated to include the aerial fertilization effect of the historical increase in the air's CO2 content.
Introducing their work on the subject, Bonal et al. (2011) write that "the impact of global change during the last century on the biology of tropical rainforest trees is largely unknown," but they say that "an increase in tree radial growth increment over recent decades in Amazonian tropical rainforests has been observed, leading to increased above-ground biomass at most study sites," citing the studies of Phillips et al. (1998, 2009) and Malhi et al. (2004) in this regard, while subsequently noting that "the stimulating impact on photosynthesis of increased CO2 concentrations in the air (Ca) could explain these growth patterns (Lloyd and Farquhar, 2008)."
In further investigating this phenomenon, Bonal et al. additionally assessed the impacts of historical environmental changes on leaf morphological (stomatal density, stomatal surface, leaf mass per unit area) and physiological traits (carbon isotope composition, δ13Cleaf, and discrimination, Δ13Cleaf, oxygen isotope composition, δ18Oleaf) of two tropical rainforest species (Dicorynia guianensis; Humiria balsamifera) that are abundant in the Guiana shield (Northern Amazonia)," working with leaf samples from different international herbariums that covered a 200-year time period (AD 1790-2004). So what did they learn?
The eleven researchers say their study revealed "a clear response of leaf physiological characteristics to increasing Ca for both species," consistent with the findings of previous studies "from different ecosystems (Penuelas and Azcon-Bieto, 1992; Beerling et al., 1993; Van de Water et al., 1994; Pedicino et al., 2002; Penuelas et al., 2008), and with data from tree rings in Europe (Bert et al., 1997; Duquesnay et al., 1998; Saurer et al., 2004), Africa (Gebrekirstos et al., 2009) and in tropical rainforests (Hietz et al., 2005; Silva et al., 2009; Nock et al., 2011)." More specifically, they say their results pointed to "an increase in water-use efficiency over recent decades of about 23.1 and 26.6% for Humiria and Dicorynia, respectively," driven mostly by increases in leaf photosynthesis. And they indicate that "the range of change in water-use efficiency for these two species was consistent with many results observed not only in tropical forests (Hietz et al., 2005; Nock et al., 2011), but in boreal (Saurer et al., 2004) and temperate forests (Francey and Farquhar, 1982; Penuelas and Azcon-Bieto, 1992; Bert et al., 1997; Duquesnay et al., 1998)." Bonal et al. further state that the responses of the two tree species they studied to increasing Ca appear to be "simply related to the availability of CO2 in the air (fertilization effect)," and they say that "this trend seems to be consistent with recent tree growth patterns in the Amazonian region," and indeed it is, as plants exposed to elevated CO2 concentrations are likely to lose less water via transpiration. Hence, the amount of carbon gained per unit of water lost per unit leaf area -- or water-use efficiency -- tends to increase dramatically as the air's CO2 content rises. As a result, at higher CO2 concentrations, plants can better cope under conditions of drought, thereby vastly improving their productivity and growth as opposed to conditions experienced under lower CO2.
This situation is further illustrated by Silva et al. (2009), who, working with various types of tree-ring data obtained from A. angustifolia trees growing in both forest and grassland sites in southern Brazil, compared changes in intrinsic water use efficiency - iWUE, defined as the ratio of the rate of CO2 assimilation by the trees' needles to their stomatal conductance - with concomitant historical changes in temperature, precipitation and atmospheric CO2 concentration that occurred over the past century. And based on this analysis, the four researchers found that during the past several decades, "iWUE increased over 30% in both habitats" (see Figure 27), and that "this increase was highly correlated with increasing levels of CO2 in the atmosphere." Over this latter period, however, tree growth remained rather stable, due to lower-than-normal precipitation and higher-than-normal temperatures, which would normally tend to depress the growth of this species, as Katinas and Crisci (2008) describe A. angustifolia as being "intolerant of dry seasons and requiring cool temperatures." As a result, Silva et al. concluded that the "climatic fluctuations during the past few decades," which would normally be expected to have been deleterious to the growth of A. angustifolia, seem to have had their growth-retarding effects "compensated by increases in atmospheric CO2 and changes [i.e., increases] in iWUE."
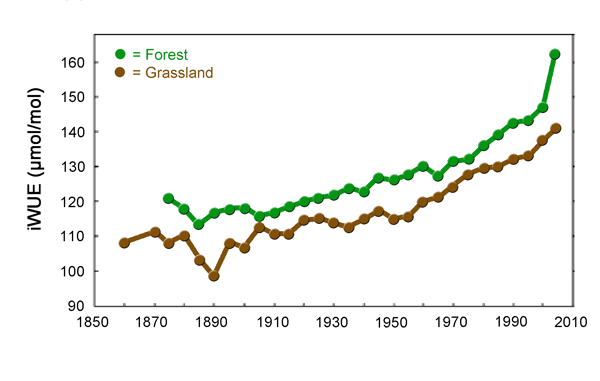
Figure 27. Intrinsic water-use efficiency (iWUE) in Araucaria angustifolia trees established into forest and grassland in southern Brazil (means of individuals per site in 5-years total values). Adapted from Silva et al. (2009).
Also working along this line of examination was Phillips et al. (2009), who set out to investigate what negative effect a severe drought might have on South America's surprisingly-spry-for-its-age tropical mega-forest, especially a drought of the type that the world's climate alarmists predict will occur if anthropogenic CO2 emissions are not significantly abated. More specifically, what the international team of scientists wanted to know was whether such a decline in the availability of water might wipe out the super ecosystem's biomass gains of prior decades, thereby fulfilling one of the climate alarmists' worst-case catastrophic scenarios.
Focusing their attention on the Amazonian drought of 2005, which they describe as "one of the most intense droughts of the past 100 years," as well as "a possible analog of future events," the 66 researchers (who had monitored a host of forest plots across the Amazon basin over the prior quarter-century) utilized tree diameter, wood density, and allometric models to compute the basin's woody biomass at each time of measurement, both before and after the drought, deriving the results that are plotted in the Figure 28 below.
As may readily be seen from these real-world measurement-based results, the great Amazonian drought of 2005 resulted in only a slight hiatus in the strong upward trend of tree biomass accumulation that was exhibited over the prior two decades, which had occurred, as Phillips et al. note, through a multi-decadal period spanning both wet and dry episodes, the latter of which are not even detectable in their wood biomass data. Hence, it would appear that although extremely severe drought conditions can indeed bring a halt to biomass accumulation in old growth tropical forests -- and sometimes even lead to minor reductions in biomass due to selective tree mortality -- the vast majority of the aged trees are able to regain their photosynthetic prowess and add to their prior store of biomass once the moisture stress subsides, thanks in large measure to the enhanced growth (Lin et al., 1998) and water use efficiency (Hietz et al., 2005) that are experienced by nearly all woody plants as the air's CO2 content rises.
Additional support for this attribution is provided by the work of Lloyd and Farquhar (2008), who concluded that "the magnitude and pattern of increases in forest dynamics across Amazonia observed over the last few decades are consistent with a CO2-induced stimulation of tree growth," while still more support for the premise comes from the work of Phillips et al. (2008), who concluded that the simplest explanation for the phenomenon is that "improved resource availability has increased net primary productivity, in turn increasing growth rates," and who further note that "the only change for which there is unambiguous evidence that the driver has widely changed and that such a change should accelerate forest growth is the increase in atmospheric CO2," because of "the undisputed long-term increase in [its] concentration, the key role of CO2 in photosynthesis, and the demonstrated effects of CO2 fertilization on plant growth rates."
In light of the voluminous and undeniable real-world observations reported in the studies described above, it must be acknowledged that where tropical forests have not been decimated by the direct destructive actions of man, such as the felling and burning of trees, forest productivity has been growing ever greater with the passing of time, rising hand-in-hand with the increasing CO2 content of the air; and it has been doing so in spite of all concomitant changes in atmospheric, soil, and water chemistry, as well as "dreaded" 20th-century global warming. Real-world evidence also suggests that the anthropogenic-induced increase in the air's CO2 content is primarily responsible for this beneficent state of affairs, which further suggests that if humanity will but cease its direct physical assaults upon Earth's tropical forests, there is nothing to fear about their future well-being but ill-founded fear itself.




