| Tweet | Follow @co2science |
Paper Reviewed
Ross, C.L., DeCarlo, T.M. and McCulloch, M.T. 2019. Environmental and physiochemical controls on coral calcification along a latitudinal temperature gradient in Western Australia. Global Change Biology 25: 431-447.
The global increase in the atmosphere's CO2 content has been hypothesized to possess the potential to harm coral reefs directly. By inducing changes in ocean water chemistry that can lead to reductions in the calcium carbonate saturation state of seawater (Ω), it has been predicted that elevated levels of atmospheric CO2 may reduce rates of coral calcification, possibly leading to slower-growing -- and, therefore, weaker -- coral skeletons, and in some cases even death.
As we have previously pointed out on our website, however (see More Proof of a Biological Control on Coral Calcification, The End of the Ocean Acidification Scare for Corals, and A Coral's Biological Control of its Calcifying Medium to Favor Skeletal Growth), such projections often fail to account for the fact that coral calcification is a biologically mediated process, and that out in the real world, living organisms tend to find ways to meet and overcome the many challenges they face; and coral calcification in response to ocean acidification is no exception.
The latest study to demonstrate this fact comes from Ross et al. (2019). Conducting geochemical analyses (using boron isotopes, elemental systematics and Raman spectroscopy) the three Australian scientists investigated seasonal changes in the full calcifying fluid chemistry (i.e., pHcf, DICcf, [CO32-]cf, [Ca2+]cf and Ωcf) of seven coral species from three reef locations off the coast of Western Australia, which locations spanned a latitude range of 11° and a temperature range of approximately 11°C.
In discussing their findings, Ross et al. report the following, as illustrated in the figure below: (1) corals biologically upregulated the pH of their calcifying fluid from 0.20 to 0.55 pH unit above the pH of the surrounding seawater, (2) DICcf was "substantially elevated relative to ambient seawater by a factor of 1.4-2.3 depending on location, season and species," (3) carbonate ion concentration of the calcifying fluid ([CO32-]cf) was also significantly higher (2 to 5 times) compared to the concentration in normal seawater, (4) [Ca2+]cf ranged from a factor of 0.68 to 1.24 above normal seawater, and (5) the calcium carbonate saturation state of the calcifying fluid (Ωcf) was three to four times higher than ambient seawater and ranged from 9.5 to 12.5, depending on species and location.
In commenting on these findings and the relationships and associations between these coral calcifying fluid variables, the three Australian researchers ultimately concluded that "changes in net coral calcification rates are primarily driven by pHcf and carbonate ion concentration [CO32-]cf in conjunction with temperature and DICcf," while adding that "coral pHcf varies with latitudinal and seasonal changes in temperature and works together with the seasonally varying DICcf to optimize [CO32-]cf at species-dependent levels.
In light of these many findings, Ross et al. conclude their results indicate that "corals shift their pHcf to adapt and/or acclimatize to their localized thermal regimes," which "biological response is likely to have critical implications for predicting the future of coral reefs under CO2-driven warming and acidification."
Indeed, and those implications suggest that coral calcification is a lot more stable than many climate alarmists assume. Such biological control further suggests that corals are likely well-equipped to continue building their skeletons under projections of future increases in oceanic temperature and seawater pH declines.
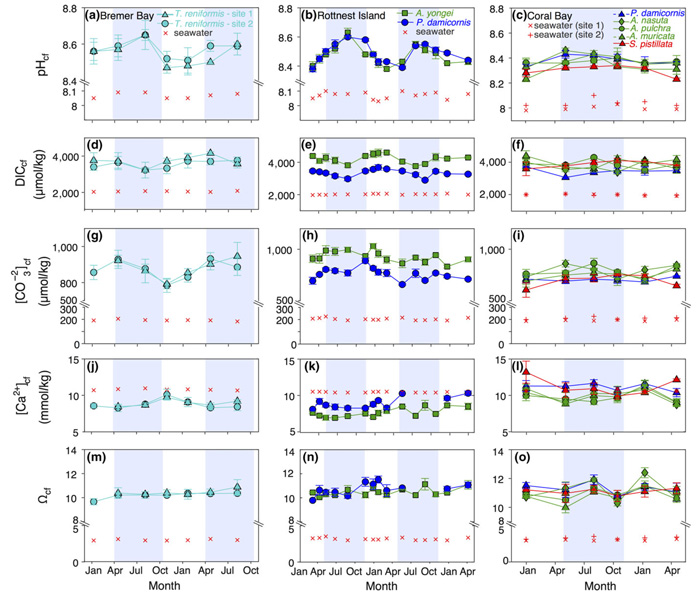
Figure 1. Time series of (a-c) pHcf, (d-f) DICcf, (g-i) [CO32-]cf, (j-l) [Ca2+]cf and (m-o) Ωcf for the coral calcifying fluid (cf). Equivalent values for seawater are shown with red crosses. Values represent mean ± 1 SE for calcifying fluid (cf) parameters. No shading denotes summer, and light blue shading denotes winter. Study locations from left to right are Bremer Bay, Rottnest Island, and Coral Bay (Ningaloo Reef). Source: Ross et al. (2019).




