| Tweet | Follow @co2science |
Paper Reviewed
DeCarlo, T.M., Comeau, S., Cornwall, C.E. and McCulloch, M.T. 2018. Coral resistance to ocean acidification linked to increased calcium at the site of calcification. Proceedings of the Royal Society B 285: 20180564, http://dx.doi.org/10.1098/rspb.2018.0564.
The global increase in the atmosphere's CO2 content has been hypothesized to possess the potential to harm coral reefs directly. By inducing changes in ocean water chemistry that can lead to reductions in the calcium carbonate saturation state of seawater (Ωsw), it has been predicted that elevated levels of atmospheric CO2 may reduce rates of coral calcification, possibly leading to slower-growing -- and, therefore, weaker -- coral skeletons, and in some cases even death. However, such projections often fail to account for the fact that coral calcification is a biologically mediated process, and that out in the real world, living organisms tend to find ways to meet and overcome the many challenges they face (see McCulloch et al. 2017; Raybaud et al. 2017; Ross et al., 2017).
The latest study to demonstrate that fact is that of DeCarlo et al. (2018). Using two novel geochemical techniques based on boron systematics and Raman spectroscopy, the four scientists derived estimates of calcium concentrations within the coral calcifying fluid ([Ca2+]cf) of two coral species (Acropora youngei and Pocillopora damicornis) that they used to investigate "the response of [Ca2+]cf to seawater pH and its potential role in controlling the calcification sensitivity to ocean acidification."
The results of their study suggest that both corals were able to control their [Ca2+]cf, elevating it as much as 25% above the calcium concentration of seawater in seawater of lower pH (Figure 1a), albeit this ability diminished for A. youngei at the lowest seawater pH (7.63) tested by the authors. In commenting on this finding, De Carlo et al. say that the ability of P. damicornis to control [Ca2+]cf resulted in its maintaining a similar aragonite saturation state within the coral calcifying fluid (Figure 1c) to that of A. youngei, despite having a lower [CO32-]cf (Figure 1b), which allowed P. damicornis to maintain its level of calcification at lower seawater pH (pHsw), whereas A. youngei did not. Thus, the researchers conclude that "some corals have cellular mechanisms in place to modulate [Ca2+]cf, and our results demonstrate that resistant species such as Pocillopora can upregulate these processes to resist the effects of ocean acidification and to maintain normal calcification rates even at low pHsw."
The significance of the above findings is noted in the fact that, as stated by Decarlo et al., "most models of coral calcification rely on the assumption that [the] aragonite saturation state is controlled by [CO32-]cf and that variability in [Ca2+]cf is negligible." Clearly, as indicated above that assumption is not correct; and it is further evident in viewing Figure 2, which plots the sensitivity of the aragonite saturation state (ΩAr) of the coral calcifying fluid to both [CO32-]cf and [Ca2+]cf. As illustrated, there is no significant correlation between ΩAr of the coral calcifying fluid and [CO32-]cf, whereas ΩAr of the coral calcifying fluid is positively correlated with [Ca2+]cf.
In light of these many findings and considerations, DeCarlo et al. conclude the abstract of their paper by writing "although the role of [Ca2+]cf in driving calcification has often been neglected, increasing [Ca2+]cf may be a key mechanism enabling more resistant corals to cope with ocean acidification and continue to build calcium carbonate skeletons in a high-CO2 world." And that great news suggests that horror stories of corals being driven to extinction because of ocean acidification are far from certain -- if they contain any truth at all!
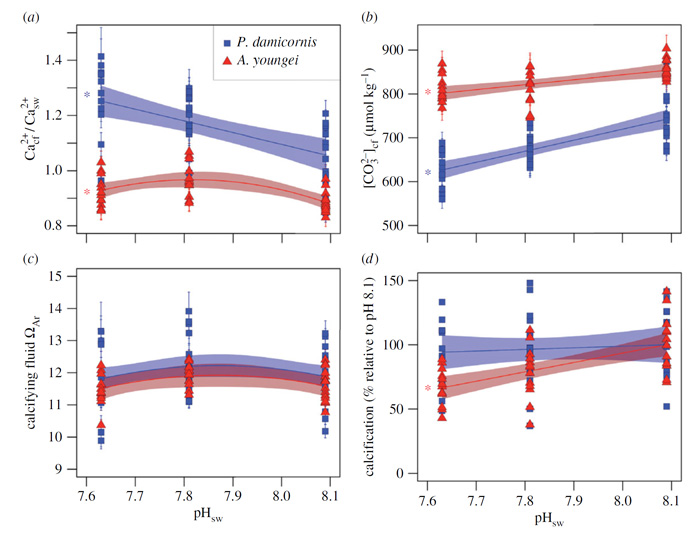
Figure 1. Sensitivity of calcifying fluid chemistry and calcification to seawater pH. (a) Ca2+cf /Ca2+sw, (b) [CO32-]cf, (c) ΩAr and (d ) calcification responses to pHsw treatments for both P. damicornis (blue squares) and A. youngei (red triangles). Bounded lines indicate regression fits ± 1 s.e. Colored asterisks indicate which regressions are statistically significant ( p < 0.01). Error bars on individual points represent 1 s.e.m. Source: DeCarlo et al. (2018).
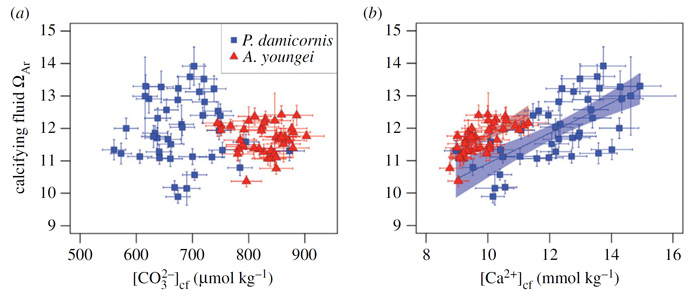
Figure 2. Relative influences on coral calcifying fluid ΩAr. Sensitivity of ΩAr to (a) [CO32-]cf and (b) [Ca2+]cf. Source: DeCarlo et al. (2018).
References
McCulloch, M.T., D'Olivo, J.P., Falter, J., Holcomb, M. and Trotter, J.A. 2017. Coral calcification in a changing world and the interactive dynamics of pH and DIC upregulation. Nature Communications 8: 15686, DOI:10.1038/ncomms15686.
Raybaud, V., Tambutté, S., Ferrier-Pagès, C., Reynaud, S., Venn, A.A., Tambutté, É., Nival, P. and Allemand, D. 2017. Computing the carbonate chemistry of the coral calcifying medium and its response to ocean acidification. Journal of Theoretical Biology 424: 26-36.
Ross, C.L., Falter, J.L. and McCulloch, M.T. 2017. Active modulation of the calcifying fluid carbonate chemistry (δ11B, B/Ca) and seasonally invariant coral calcification at sub-tropical limits. Scientific Reports 7: 13830, DOI:10.1038/s41598-017-14066-9.
Posted 10 August 2018



