Volume 12, Number 22: 3 June 2009
There is considerable current concern that the ongoing rise in the air's CO2 content is causing a significant drop in the pH of the world's oceans in response to their absorption of a large fraction of each year's anthropogenic CO2 emissions. It has been estimated, for example, that the globe's seawater has been acidified (actually made less basic) by about 0.1 pH unit relative to what it was in pre-industrial times; and model calculations imply an additional 0.7-unit drop by the year 2300 (Caldeira and Wickett, 2003), which decline is hypothesized to cause great harm to calcifying marine life such as corals. But just how valid are these claims?
Whenever the results of theoretical calculations are proposed as the basis for a crisis of some kind or other, it is always good to compare their predictions against what is known about the phenomenon in the real world. In the case of oceanic pH, for example, Liu et al. (2009) write in an important new paper that "the history of ocean pH variation during the current interglacial (Holocene) remains largely unknown," and that it "would provide critical insights on the possible impact of acidification on marine ecosystems." Hence, they set about to provide just such a context.
Working with eighteen samples of fossil and modern Porites corals recovered from the South China Sea, the nine researchers employed 14C dating using the liquid scintillation counting method, along with positive thermal ionization mass spectrometry to generate high precision δ11B (boron) data, from which they reconstructed the paleo-pH record of the past 7000 years that is depicted in the figure below.
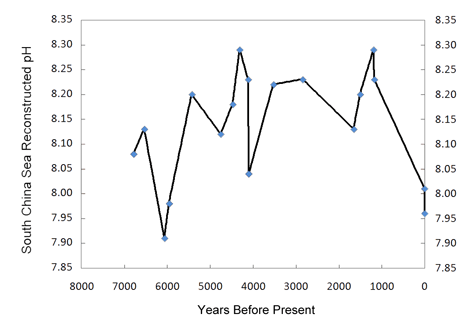
Reconstructed pH history of the South China Sea. Created from Table 1 of Liu et al. (2009).
As can be seen from this figure, there is nothing unusual, unnatural or unprecedented about the two most recent pH values. They are neither the lowest of the record, nor is the decline rate that led to them the greatest of the record. Hence, there is no compelling reason to believe they were influenced in any way by the nearly 40% increase in the air's CO2 concentration that has occurred to date over the course of the Industrial Revolution. As for the prior portion of the record, Liu et al. note that there is also "no correlation between the atmospheric CO2 concentration record from Antarctica ice cores and δ11B-reconstructed paleo-pH over the mid-late Holocene up to the Industrial Revolution."
Further enlightenment comes from the earlier work of Pelejero et al. (2005), who developed a more refined history of seawater pH spanning the period 1708-1988 (depicted in the figure below), based on δ11B data obtained from a massive Porites coral from Flinders Reef in the western Coral Sea of the southwestern Pacific. These researchers also found that "there is no notable trend toward lower δ11B values." Instead, they discovered that "the dominant feature of the coral δ11B record is a clear interdecadal oscillation of pH, with δ11B values ranging between 23 and 25 per mil (7.9 and 8.2 pH units)," which they say "is synchronous with the Interdecadal Pacific Oscillation."

Reconstructed pH history of Flinders Reef of the Western Coral Sea of the Southwestern Pacific. Adapted from Pelejero et al. (2005).
Going one step further, Pelejero et al. also compared their results with coral extension and calcification rates obtained by Lough and Barnes (1997) over the same 1708-1988 time period; and as best we can determine from their graphical representations of these two coral growth parameters, extension rates over the last 50 years of this period were about 12% greater than they were over the first 50 years, while calcification rates were approximately 13% greater over the last 50 years.
Most recently, Wei et al. (2009) derived the pH history of Arlington Reef (off the north-east coast of Australia) that is depicted in the figure below. As can be seen there, there was a ten-year pH minimum centered at about 1935 (which obviously was not CO2-induced) and a shorter more variable minimum at the end of the record (which also was not CO2-induced); and apart from these two non-CO2-related exceptions, the majority of the data once again fall within a band that exhibits no long-term trend, such as would be expected to have occurred if the gradual increase in atmospheric CO2 concentration since the inception of the Industrial Revolution were truly making the global ocean less basic.

Reconstructed pH history of Arlington Reef off the northeast coast of Australia. Adapted from Wei et al. (2009).
In light of these several diverse and independent assessments of the two major aspects of the ocean acidification hypothesis -- a CO2-induced decline in oceanic pH that leads to a concomitant decrease in coral growth rate -- it would appear that the catastrophe conjured up by the world's climate alarmists is but a wonderful work of fiction.
Sherwood, Keith and Craig Idso
References
Caldeira, K. and Wickett, M.E. 2003. Anthropogenic carbon and ocean pH. Nature 425: 365.
Liu, Y., Liu, W., Peng, Z., Xiao, Y., Wei, G., Sun, W., He, J. Liu, G. and Chou, C.-L. 2009. Instability of seawater pH in the South China Sea during the mid-late Holocene: Evidence from boron isotopic composition of corals. Geochimica et Cosmochimica Acta 73: 1264-1272.
Lough, J.M. and Barnes, D.J. 1997. Several centuries of variation in skeletal extension, density and calcification in massive Porites colonies from the Great Barrier Reef: A proxy for seawater temperature and a background of variability against which to identify unnatural change. Journal of Experimental and Marine Biology and Ecology 211: 29-67.
Pelejero, C., Calvo, E., McCulloch, M.T., Marshall, J.F., Gagan, M.K., Lough, J.M. and Opdyke, B.N. 2005. Preindustrial to modern interdecadal variability in coral reef pH. Science 309: 2204-2207.
Wei, G., McCulloch, M.T., Mortimer, G., Deng, W. and Xie, L. 2009. Evidence for ocean acidification in the Great Barrier Reef of Australia. Geochimica et Cosmochimica Acta 73: 2332-2346.




