Climate alarmists contend that rising air temperatures - which they claim are caused by rising atmospheric CO2 concentrations that are caused by rising anthropogenic CO2 emissions - will wreck havoc with earth's biosphere, both on land and at sea. In light of what they call the unprecedented increases in these two parameters over the course of the 20th century, therefore, one might reasonably expect there would already be signs of a major negative impact on oceanic productivity. In this section we thus search for evidence of such a downturn in the productivity of earth's seas, but we begin with a study that takes a much longer view of the subject.
Elderfield and Rickaby (2000) note that the typically low atmospheric CO2 concentrations of glacial periods have generally been attributed to an increased oceanic uptake of CO2, "particularly in the southern oceans." However, the assumption that intensified phytoplanktonic photosynthesis may have stimulated CO2 uptake rates during glacial periods has always seemed at odds with the observational fact that rates of photosynthesis are generally much reduced in environments of significantly lower-than-current atmospheric CO2 concentrations, such as typically prevail during glacial periods.
The two scientists provide a new interpretation of Cd/Ca systematics in sea water that helps to resolve this dichotomy, as it allows them to more accurately estimate surface water phosphate conditions during glacial times and thereby determine the implications for concomitant atmospheric CO2 concentrations. What they find, in their words, is that "results from the Last Glacial Maximum [LGM] show similar phosphate utilization in the subantarctic to that of today, but much smaller utilization in the polar Southern Ocean," which implies, according to Delaney (2000), that Antarctic productivity was lower at that time than it is now, but that subantarctic productivity was about the same as it has been in modern times, due perhaps to greater concentrations of bio-available iron compensating for the lower atmospheric CO2 concentrations of the LGM.
So what caused the much smaller utilization of phosphate in the polar Southern Ocean during the LGM? Noting that the area of sea-ice cover in the Southern Ocean during glacial periods may have been as much as double that of modern times, Elderfield and Rickaby suggest that "by restricting communication between the ocean and atmosphere, sea ice expansion also provides a mechanism for reduced CO2 release by the Southern Ocean and lower glacial atmospheric CO2." Hence, it is possible that phytoplanktonic productivity in the glacial Southern Ocean may well have been no higher than it is at the present time, notwithstanding the greater supply of bio-available iron typical of glacial epochs.
In the case of the interglacial period in which we currently live, Dupouy et al. (2000) say it has long been believed that N2 fixation in the world's oceans is unduly low, in consequence of the present low supply of wind-blown iron compared to that of glacial periods, and that this state of affairs leads to low phytoplanktonic productivity, even in the presence of higher atmospheric CO2 concentrations. The evidence they acquired, however, suggests that marine N2 fixation may be much greater than what has generally been thought to be the case. In particular, they note that several Trichodesmium species of N2-fixing cyanobacteria have "a nearly ubiquitous distribution in the euphotic zone of tropical and subtropical seas and could play a major role in bringing new N to these oligotrophic systems." And this feat, in their words, "could play a significant role in enhancing new production."
The importance of these findings is perhaps best appreciated in light of the findings of Pahlow and Riebesell (2000), who in studying data obtained from 1173 stations in the Atlantic and Pacific Oceans, covering the years 1947 to 1994, detected changes in Northern Hemispheric deep-ocean Redfield ratios that are indicative of increasing nitrogen availability there, which increase was concomitant with an increase in export production that has resulted in ever-increasing oceanic carbon sequestration. These investigators further suggest that the growing supply of nitrogen has its origin in anthropogenic activities that release nitrous oxides to the air. In addition, the increased carbon export may be partly a consequence of the historical increase in the air's CO2 concentration, which has been demonstrated to have the ability to enhance phytoplanktonic productivity (see Section 6.1.3.4. in this document), analogous to the way in which elevated concentrations of atmospheric CO2 enhance the productivity of terrestrial plants, including their ability to fix nitrogen.
Further elucidating the productivity-enhancing power of the ongoing rise in the air's CO2 content is the study of Pasquer et al. (2005), who employed a complex model of growth regulation of diatoms, pico/nano phytoplankton, coccolithophorids and Phaeocystis spp. by light, temperature and nutrients (based on a comprehensive analysis of literature reviews focusing on these taxonomic groups) to calculate changes in the ocean uptake of carbon in response to a sustained increase in atmospheric CO2 concentration of 1.2 ppm per year for three marine ecosystems where biogeochemical time-series of the data required for model initialization and comparison of results were readily available. These systems were (1) the ice-free Southern Ocean Time Series station KERFIX (50°40'S, 68°E) for the period 1993-1994 (diatom-dominated), (2) the sea-ice associated Ross Sea domain (76°S, 180°W) of the Antarctic Environment and Southern Ocean Process Study AESOPS in 1996-1997 (Phaeocystis-dominated), and (3) the North Atlantic Bloom Experiment NABE (60°N, 20°W) in 1991 (coccolithophorids). Their results, in their words, "show that at all tested latitudes the prescribed increase of atmospheric CO2 enhances the carbon uptake by the ocean." Indeed, we calculate from their graphical presentations that (1) at the NABE site a sustained atmospheric CO2 increase of 1.2 ppm per year over a period of eleven years increases the air-sea CO2 flux in the last year of that period by approximately 17%, (2) at the AESOPS site the same protocol applied over a period of six years increases the air-sea CO2 flux by about 45%, and (3) at the KERFIX site it increases the air-sea CO2 flux after nine years by about 78%. Although the results of this interesting study based on the complex SWAMCO model of Lancelot et al. (2000), as modified by Hannon et al. (2001), seem overly large, they highlight the likelihood that the ongoing rise in the air's CO2 content may be having a significant positive impact on ocean productivity and the magnitude of the ocean carbon sink.
But what about increasing temperatures? Sarmiento et al. (2004) conducted a massive computational study that employed six coupled climate model simulations to determine the biological response of the global ocean to the climate warming they simulated from the beginning of the Industrial Revolution to the year 2050. Based on vertical velocity, maximum winter mixed-layer depth and sea-ice cover, they defined six biomes and calculated how their surface geographies would change in response to their calculated changes in global climate. Next, they used satellite ocean color and climatological observations to develop an empirical model for predicting surface chlorophyll concentrations from the final physical properties of the world's oceans as derived from their global warming simulations, after which they used three primary production algorithms to estimate the response of oceanic primary production to climate warming based on their calculated chlorophyll concentrations. When all was said and done, the thirteen scientists from Australia, France, Germany, Russia, the United Kingdom and the United States arrived at a global warming-induced increase in global ocean primary production that ranged from 0.7 to 8.1%, which is a far, far cry from the disastrous consequences routinely predicted by climate alarmists.
So what do real-world measurements of oceanic productivity reveal? Goes et al. (2005) analyzed seven years (1997-2004) of satellite-derived ocean color data pertaining to the Arabian Sea, as well as associated sea surface temperatures (SSTs) and winds. They report that for the region located between 52 to 57°E and 5 to 10°N, "the most conspicuous observation was the consistent year-by-year increase in phytoplankton biomass over the 7-year period." This phenomenon was so dramatic that by the summer of 2003, in their words, "chlorophyll a concentrations were >350% higher than those observed in the summer of 1997." They also report that the increase in chlorophyll a was "accompanied by an intensification of sea surface winds, in particular of the zonal (east-to-west) component," noting that these "summer monsoon winds are a coupled atmosphere-land-ocean phenomenon, whose strength is significantly correlated with tropical SSTs and Eurasian snow cover anomalies on a year-to-year basis." More specifically, they say that "reduced snow cover over Eurasia strengthens the spring and summer land-sea thermal contrast and is considered to be responsible for the stronger southwest monsoon winds." In addition, they state that "the influence of southwest monsoon winds on phytoplankton in the Arabian Sea is not through their impact on coastal upwelling alone but also via the ability of zonal winds to laterally advect newly upwelled nutrient-rich waters to regions away from the upwelling zone." Their final conclusion about the matter is that "escalation in the intensity of summer monsoon winds, accompanied by enhanced upwelling and an increase of more than 350% in average summertime phytoplankton biomass along the coast and over 300% offshore, raises the possibility that the current warming trend of the Eurasian landmass is making the Arabian Sea more productive."
To the north and west on the other side of Eurasia, Marasovic et al. (2005) analyzed monthly observations of basic hydrographic, chemical and biological parameters, including primary production, that had been made since the 1960s at two oceanographic stations, one near the coast (Kastela Bay) and one in the open sea. They found that mean annual primary production in Kastela Bay averaged about 430 mg C m-2 d-1 over the period 1962-72, exceeded 600 mg C m-2 d-1 over the period 1972-82, and rose to over 700 mg C m-2 d-1 over the period 1982-96, accompanied by a similar upward trend in percent oxygen saturation of the surface water. The initial value of primary production in the open sea was much less (approximately 150 mg C m-2 d-1), but it began to follow the upward trend of the Kastela Bay data after about one decade. Marasovic et al. thus concluded that "even though all the relevant data indicate that the changes in Kastela Bay are closely related to an increase of anthropogenic nutrient loading, similar changes in the open sea suggest that primary production in the Bay might, at least partly, be due to global climatic changes," which, in their words, are "occurring in the Mediterranean and Adriatic Sea open waters" and may be directly related to "global warming of air and ocean," since "higher temperature positively affects photosynthetic processes."
Raitsos et al. (2005) investigated the relationship between Sea-viewing Wide Field-of-view Sensor (SeaWiFS) chlorophyll-a measurements in the Central Northeast Atlantic and North Sea (1997-2002) and simultaneous measurements of the Phytoplankton Color Index (PCI) collected by the Continuous Plankton Recorder survey, which is an upper-layer plankton monitoring program that has operated in the North Sea and North Atlantic Ocean since 1931. By developing a relationship between the two data bases over their five years of overlap, they were able to produce a Chl-a history for the Central Northeast Atlantic and North Sea for the period 1948-2002. Of this record they say that "an increasing trend is apparent in mean Chl-a for the area of study over the period 1948-2002." They also say "there is clear evidence for a stepwise increase after the mid-1980s, with a minimum of 1.3mg m-3 in 1950 and a peak annual mean of 2.1 mg m-3 in 1989 (62% increase)." Alternatively, it is possible that the data represent a more steady long-term upward trend upon which is superimposed a decadal-scale oscillation. In a final comment on their findings, they note that "changes through time in the PCI are significantly correlated with both sea surface temperature and Northern Hemisphere temperature," citing Beaugrand and Reid (2003).
In a contemporaneous study, Antoine et al. (2005) applied revised data-processing algorithms to two ocean-sensing satellites, the Coastal Zone Color Scanner (CZCS) and SeaWiFS, over the periods 1979-1986 and 1998-2002, respectively, to provide an analysis of the decadal changes in global oceanic phytoplankton biomass. Results of the analysis showed "an overall increase of the world ocean average chlorophyll concentration by about 22%" over the two decades under study, which results are truly impressive considering (a) the significant increase in the air's CO2 content over the period of study - the continuation of which climate alarmists claim will eventually prove the undoing of all types of aquatic life - and (b) the supposedly unprecedented (over the past two millennia) rate of rise of the mean temperature of the globe - which radical environmentalists claim will do the same.
Dropping down to the Southern Ocean, Hirawake et al. (2005) analyzed chlorophyll a data obtained from Japanese Antarctic Research Expedition cruises made by the Fuji and Shirase ice-breakers between Tokyo and Antarctica from 15 November to 28 December of nearly every year between 1965 and 2002 in a study of interannual variations of phytoplankton biomass, calculating results for the equatorial region between 10°N and 10°S, the Subtropical Front (STF) region between 35°S and 45°S, and the Polar Front (PF) region between 45°S and 55°S. They report that an increase in chl a was "recognized in the waters around the STF and the PF, especially after 1980 around the PF in particular," and that "in the period between 1994 and 1998, the chl a in the three regions exhibited rapid gain simultaneously." They also say "there were significant correlations between chl a and year through all of the period of observation around the STF and PF, and the rates of increase are 0.005 and 0.012 mg chl a m-3 y-1, respectively." In addition, they report that the satellite data of Gregg and Conkright (2002) "almost coincide with our results." In commenting on these findings, the Japanese scientists say that "simply considering the significant increase in the chl a in the Southern Ocean, a rise in the primary production as a result of the phytoplankton increase in this area is also expected."
Also working in the Southern Hemisphere, Sepulveda et al. (2005) presented "the first reconstruction of changes in surface primary production during the last century from the Puyuhuapi fjord in southern Chile, using a variety of parameters (diatoms, biogenic silica, total organic carbon, chlorins, and proteins) as productivity proxies." Noting that the fjord is located in "a still-pristine area," they say it is "suitable to study changes in past export production originating from changes in both the paleo-Patagonian ice caps and the globally important Southern Ocean."
The analysis revealed that the productivity of the Puyuhuapi fjord "was characterized by a constant increase from the late 19th century to the early 1980s, then decreased until the late 1990s, and then rose again to present-day values." For the first of these periods (1890-1980), they additionally report that "all proxies were highly correlated (r > 0.8, p < 0.05)," and that "all proxies reveal an increase in accumulation rates." From 1980 to the present, however, the pattern differed among the various proxies; and the researchers say that "considering that the top 5 cm of the sediment column (~10 years) are diagenetically active, and that bioturbation by benthic organisms may have modified and mixed the sedimentary signal, paleo-interpretation of the period 1980-2001 must be taken with caution." Consequently, there is substantial solid evidence that, for the first 90 years of the 111-year record, surface primary production in the Puyuhuapi fjord rose dramatically, while with lesser confidence it appears to have leveled out over the past two decades.
Thus, in spite of climate-alarmist contentions that the "unprecedented" increases in mean global air temperature and CO2 concentration experienced since the inception of the Industrial Revolution have been bad for the biosphere, Sepulveda et al. presented yet another case of an ecosystem apparently thriving in such conditions. Nevertheless, claims of impending ocean productivity declines have not ceased, and many alarmists have signaled out the study of Behrenfeld et al. (2006) in support of their claims, for which the supporting data looked almost halfway decent ... initially, at least.
Working with NASA's Sea-viewing Wide Field-of-view Sensor (Sea WiFS), the team of ten U.S. scientists calculated monthly changes in net primary production (NPP) from similar changes in upper-ocean chlorophyll concentrations detected from space over the past decade (see figure below). They report that this period was dominated by an initial NPP increase (represented by their initial ascending straight line) of 1,930 teragrams of carbon per year (Tg C yr-1), which they attributed to the significant cooling of "the 1997 to 1999 El Niño to La Niña transition," and they note that this increase was "followed by a prolonged decrease [represented by their subsequent descending straight line] averaging 190 Tg C yr-1," which they attributed to subsequent warming.
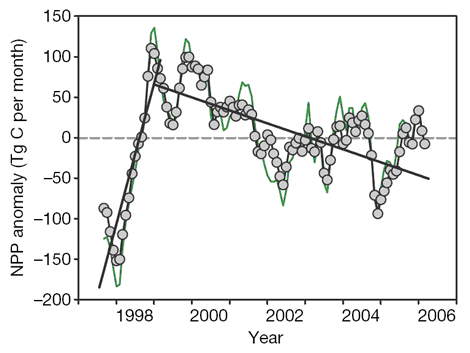
Figure Caption: Monthly anomalies of global NPP (green line) plus similar results for the permanently stratified ocean regions of the world (grey circles and black line), adapted from Behrenfeld et al. (2006).
The means by which changing temperatures were claimed by the researchers to have driven the two sequential linear-fit trends in NPP is based on their presumption that a warming climate increases the density contrast between warmer surface waters and cooler underlying nutrient-rich waters, so that the enhanced stratification that occurs with warming "suppresses nutrient exchange through vertical mixing," which decreases NPP by reducing the supply of nutrients to the surface waters where photosynthesizing phytoplankton predominantly live. In contrast, the ten scientists suggest that "surface cooling favors elevated vertical exchange," which increases NPP by enhancing the supply of nutrients to the ocean's surface waters, which are more frequented by phytoplankton than are under-lying waters, due to light requirements for photosynthesis.
This is all well and good; but it is informative to note that from approximately the middle of 2001 to the end of the data series in early 2006 (which interval accounts for more than half of the entire data record), there has been, if anything, a slight increase in global NPP. Does this observation mean there has been little to no net global warming since mid-2001? Or does it mean that the global ocean's mean surface temperature actually cooled a bit over the last five years? Any way you look at it, to paraphrase Simon and Garfunkel, climate alarmists lose, for neither alternative is what one would expect if the earth was truly racing headlong into a CO2-induced climate inferno, as the world's climate alarmists are continually ranting and raving it is doing ... and doing, as they like to suggest, with an unprecedented vengeance.
On the other hand, the relationship between global warming and oceanic productivity may not be nearly as strong as what Behrenfeld et al. have suggested; and they actually leave themselves some significant "wiggle room" in this regard, saying "modeling studies suggest that shifts in ecosystem structure from climate variations may be as [important as] or more [our italics] important than the alterations in bulk integrated properties reported here," noting that some "susceptible ecosystem characteristics" that might be so shifted include "taxonomic composition, physiological status, and light absorption by colored dissolved organic material." Hence, it is possible that given enough time, the types of phenomena Behrenfeld et al. describe as possibly resulting in important "shifts in ecosystem structure" could well compensate, or even over-compensate, for what might initially appear to be negative warming-induced consequences.
Another reason for not concluding too much from the oceanic NPP data set of Behrenfeld et al. is that it may be of too short a duration to reveal what might be occurring on a much longer timescale throughout the world's oceans, or that its position in time may be such that it does not allow the detection of far greater short-term changes of the opposite sign that may have occurred a few years earlier or that might occur in the near future.
Consider, for example, the fact that the central regions of the world's major oceans were long thought to be essentially vast biological deserts (Ryther, 1969), but that several studies of primary photosynthetic production conducted in those regions over the 1980s (Shulenberger and Reid, 1981; Jenkins, 1982; Jenkins and Goldman, 1985; Reid and Shulenberger, 1986; Marra and Heinemann, 1987; Laws et al., 1987; Venrick et al., 1987; Packard et al., 1988) yielded results that suggested marine productivity at that time was twice or more as great as it likely was for a long time prior to 1969, causing many of that day to speculate that "the ocean's deserts are blooming" (Kerr, 1986).
Of even greater interest, perhaps, is the fact that over this particular period of time (1970-1988), the data repository of Jones et al. (1999) indicates the earth experienced a (linear-regression-derived) global warming of 0.333°C, while the data base of the Global Historical Climatology Network indicates the planet experienced a similarly-calculated global warming of 0.397°C. The mean of these two values (0.365°C) is nearly twice as great as the warming that occurred over the post-1999 period studied by Behrenfeld et al.; yet this earlier much larger warming (which according to the ten researchers' way of thinking should have produced a major decline in ocean productivity) was concomitant with a huge increase in ocean productivity. Consequently, it would appear that just the opposite of what Behrenfeld et al. suggest about global warming and ocean productivity is likely to be the more correct of the two opposing cause-and-effect relationships.
Moving closer to the present, Levitan et al. (2007) published a study of major significance that addresses the future of oceanic productivity under rising atmospheric CO2 concentrations. In their paper the authors note that "among the principal players contributing to global aquatic primary production, the nitrogen (N)-fixing organisms (diazotrophs) are important providers of new N to the oligotrophic areas of the oceans," and they cite several studies which demonstrate that "cyanobacterial (phototrophic) diazotrophs in particular fuel primary production and phytoplankton blooms which sustain oceanic food-webs and major economies and impact global carbon (C) and N cycling." These facts thus compelled them to examine how the ongoing rise in the air's CO2 content might impact these relationships; and they began by exploring the response of the cyanobacterial diazotroph Trichodesmium to changes in the atmosphere's CO2 concentration, choosing this particular diazotroph because it dominates the world's tropical and sub-tropical oceans in this regard, contributing over 50% of total marine N fixation.
The eight Israeli and Czech researchers grew Trichodesmium IMS101 stock cultures in YBCII medium (Chen et al., 1996) at 25°C and a 12-hour:12-hour light/dark cycle (with the light portion of the cycle in the range of 80-100 µmol photons m-2 s-1) in equilibrium with air of three different CO2 concentrations (250, 400 and 900 ppm, representing low, ambient and high concentrations, respectively), which was accomplished by continuously bubbling air of the three CO2 concentrations through the appropriate culture vessels throughout various experimental runs, each of which lasted a little over three weeks, during which time they periodically monitored a number of diazotrophic physiological processes and properties.
So what did the scientists learn? Levitan et al. report that Trichodesmium in the high CO2 treatment "displayed enhanced N fixation, longer trichomes, higher growth rates and biomass yields." In fact, they write that in the high CO2 treatment there was "a three- to four-fold increase in N fixation and a doubling of growth rates and biomass," and that the cultures in the low CO2 treatment reached a stationary growth phase after only five days, "while both ambient and high CO2 cultures exhibited exponential growth until day 15 before declining."
In discussing possible explanations for what they observed, the researchers suggest that "enhanced N fixation and growth in the high CO2 cultures occurs due to reallocation of energy and resources from carbon concentrating mechanisms required under low and ambient CO2." Consequently, they conclude, in their words, that "in oceanic regions, where light and nutrients such as P and Fe are not limiting, we expect the projected concentrations of CO2 to increase N fixation and growth of Trichodesmium," and that "other diazotrophs may be similarly affected, thereby enhancing inputs of new N and increasing primary productivity in the oceans." And to emphasize these points, they write in the concluding sentence of their paper that "Trichodesmium's dramatic response to elevated CO2 may consolidate its dominance in subtropical and tropical regions and its role in C and N cycling, fueling subsequent primary production, phytoplankton blooms, and sustaining oceanic food-webs."
Concluding our discussion on ocean productivity, we summarize the results of two 2008 studies conducted at opposite ends of the globe. In the first study, Arrigo et al. (2008) introduce their work by writing that "between the late 1970s and the early part of the 21st century, the extent of Arctic Ocean sea ice cover has declined during all months of the year, with the largest declines reported in the boreal summer months, particularly in September (8.6 ± 2.9% per decade)," citing the work of Serreze et al. (2007). In an effort to "quantify the change in marine primary productivity in Arctic waters resulting from recent losses of sea ice cover," the authors "implemented a primary productivity algorithm that accounts for variability in sea ice extent, sea surface temperature, sea level winds, downwelling spectral irradiance, and surface chlorophyll a concentrations," and that "was parameterized and validated specifically for use in the Arctic (Pabi et al., 2008) and utilizes forcing variables derived either from satellite data or NCEP reanalysis fields."
By means of the protocol they pursued, Arrigo et al. were able to determine that "annual primary production in the Arctic increased yearly by an average of 27.5 Tg C per year since 2003 and by 35 Tg C per year between 2006 and 2007," 30% of which total increase was attributable to decreased minimum summer ice extent and 70% of which was due to a longer phytoplankton growing season. Arrigo et al. thus conclude that if the trends they discovered continue, "additional loss of ice during Arctic spring could boost productivity >3-fold above 1998-2002 levels." Hence, they additionally state that if the 26% increase in annual net CO2 fixation in the Arctic Ocean between 2003 and 2007 is maintained, "this would represent a weak negative [our italics] feedback on climate change."
On the other side of the globe and working in the Southern Ocean, Smith and Comiso (2008) employed phytoplankton pigment assessments, surface temperature estimates, modeled irradiance, and observed sea ice concentrations -- all of which parameters were derived from satellite data -- and incorporated them into a vertically-integrated production model to estimate primary productivity trends according to the technique of Behrenfeld et al. (2002). Of this effort, the two authors say that "the resultant assessment of Southern Ocean productivity is the most exhaustive ever compiled and provides an improvement in the quantitative role of carbon fixation in Antarctic waters." So what did they find?
During the nine years (1997-2006) analyzed in the study, "productivity in the entire Southern Ocean showed a substantial and significant increase," which increase can be calculated from the graphical representation of their results as ~17% per decade. In commenting on their findings, the two researchers note that "the highly significant increase in the productivity of the entire Southern Ocean over the past decade implies that long-term changes in Antarctic food webs and biogeochemical cycles are presently occurring," which changes we might add are positive.
In light of these several real-world observations, we not only find no indications of any widespread decline in oceanic productivity over the 20th century in response to climate-alarmist-feared increases in air temperature and CO2 concentration, we see evidence that just the opposite is occurring, thanks to these very same environmental changes, which are actually proving to be beneficial.
References
Antoine, D., Morel, A., Gordon, H.R., Banzon, V.J. and Evans, R.H. 2005. Bridging ocean color observations of the 1980s and 2000s in search of long-term trends. Journal of Geophysical Research 110: 10.1029/2004JC002620.
Arrigo, K.R., van Dijken, G. and Pabi, S. 2008. Impact of a shrinking Arctic ice cover on marine primary production. Geophysical Research Letters 35: 10.1029/2008GL035028.
Beaugrand, G. and Reid, P.C. 2003. Long-term changes in phytoplankton, zooplankton and salmon related to climate. Global Change Biology 9: 801-817.
Behrenfeld, M, Maranon, E., Siegel, D.A. and Hooker, S.B. 2002. Photo-acclimation and nutrient-based model of light-saturated photosynthesis for quantifying ocean primary production. Marine Ecology Progress Series 228: 103-117.
Behrenfeld, M.J., O'Malley, R.T., Siegel, D.A., McClain, C.R., Sarmiento, J.L., Feldman, G.C., Milligan, A.J., Falkowski, P.G., Letelier, R.M. and Boss, E.S. 2006. Climate-driven trends in contemporary ocean productivity. Nature 444: 752-755.
Chen, Y.B., Zehr, J.P. and Mellon, M. 1996. Growth and nitrogen fixation of the diazotrophic filamentous nonheterocystous cyanobacterium Trichodesmium sp IMS101 in defined media: evidence for a circadian rhythm. Journal of Phycology 32: 916-923.
Delaney, P. 2000. Nutrients in the glacial balance. Nature 405: 288-291.
Dupouy, C., Neveux, J., Subramaniam, A., Mulholland, M.R., Montoya, J.P., Campbell, L., Carpenter, E.J. and Capone, D.G. 2000. Satellite captures Trichodesmium blooms in the southwestern tropical Pacific. EOS, Transactions, American Geophysical Union 81: 13, 15-16.
Elderfield, H. and Rickaby, R.E.M. 2000. Oceanic Cd/P ratio and nutrient utilization in the glacial Southern Ocean. Nature 405: 305-310.
Goes, J.I., Thoppil, P.G., Gomes, H. do R. and Fasullo, J.T. 2005. Warming of the Eurasian landmass is making the Arabian Sea more productive. Science 308: 545-547.
Gregg, W.W. and Conkright, M.E. 2002. Decadal changes in global ocean chlorophyll. Geophysical Research Letters 29: 10.1029/2002GL014689.
Hannon, E., Boyd, P.W., Silvoso, M. and Lancelot, C. 2001. Modelling the bloom evolution and carbon flows during SOIREE: implications for future in situ iron-experiments in the Southern Ocean. Deep-Sea Research II 48: 2745-2773.
Hirawake, T., Odate, T. and Fukuchi, M. 2005. Long-term variation of surface phytoplankton chlorophyll a in the Southern Ocean during 1965-2002. Geophysical Research Letters 32: 10.1029/2004GL021394.
Jenkins, W.J. 1982. Oxygen utilization rates in North Atlantic subtropical gyre and primary production in oligotrophic systems. Nature 300: 246-248.
Jenkins, W.J. and Goldman, J.C. 1985. Seasonal oxygen cycling and primary production in the Sargasso Sea. Journal of Marine Research 43: 465-491.
Jones, P.D., Parker, D.E., Osborn, T.J. and Briffa, K.R. 1999. Global and hemispheric temperature anomalies -- land and marine instrument records. In Trends: A Compendium of Data on Global Change. Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, U.S. Department of Energy, Oak Ridge, TN, USA.
Kerr, R.A. 1986. The ocean's deserts are blooming. Science 232: 1345.
Lancelot, C., Hannon, E., Becquevort, S., Veth, C. and De Baar, H.J.W. 2000. Modelling phytoplankton blooms and carbon export in the Southern Ocean: dominant controls by light and iron in the Atlantic sector in austral spring 1992. Deep Sea Research I 47: 1621-1662.
Laws, E.A., Di Tullio, G.R. and Redalje, D.G. 1987. High phytoplankton growth and production rates in the North Pacific subtropical gyre. Limnology and Oceanography 32: 905-918.
Levitan, O., Rosenberg, G., Setlik, I., Setlikova, E., Grigel, J., Klepetar, J., Prasil, O. and Berman-Frank, I. 2007. Elevated CO2 enhances nitrogen fixation and growth in the marine cyanobacterium Trichodesmium. Global Change Biology 13: 531-538.
Marasovic, I., Nincevic, Z., Kuspilic, G., Marinovic, S. and Marinov, S. 2005. Long-term changes of basic biological and chemical parameters at two stations in the middle Adriatic. Journal of Sea Research 54: 3-14.
Marra, J. and Heinemann, K.R. 1987. Primary production in the North Pacific central gyre: Some new measurements based on 14C. Deep-Sea Research 34: 1821-1829.
Pabi, S., van Dijken, G.S. and Arrigo, K.R. 2008. Primary production in the Arctic Ocean, 1998-2006. Journal of Geophysical Research 113: 10.1029/2007JC004578.
Packard, T.T., Denis, M., Rodier, M. and Garfield, P. 1988. Deep-ocean metabolic CO2 production: Calculations from ETS activity. Deep-Sea Research 35: 371-382.
Pahlow, M. and Riebesell, U. 2000. Temporal trends in deep ocean Redfield ratios. Science 287: 831-833.
Pasquer, B., Laruelle, G., Becquevort, S., Schoemann, V., Goosse, H. and Lancelot, C. 2005. Linking ocean biogeochemical cycles and ecosystem structure and function: results of the complex SWAMCO-4 model. Journal of Sea Research 53: 93-108.
Raitsos, D., Reid, P.C., Lavender, S.J., Edwards, M. and Richardson, A.J. 2005. Extending the SeaWiFS chlorophyll data set back 50 years in the northeast Atlantic. Geophysical Research Letters 32: 10.1029/2005GL022484.
Reid, J.L. and Shulenberger, E. 1986. Oxygen saturation and carbon uptake near 28°N, 155°W. Deep-Sea Research 33: 267-271.
Ryther, J.H. 1969. Photosynthesis and fish production in the sea. Science 166: 72-76.
Sarmiento, J.L., Slater, R., Barber, R., Bopp, L., Doney, S.C., Hirst, A.C., Kleypas, J., Matear, R., Mikolajewicz, U., Monfray, P., Soldatov, V., Spall, S.A. and Stouffer, R. 2004. Response of ocean ecosystems to climate warming. Global Biogeochemical Cycles 18: 10.1029/2003GB002134.
Sepulveda, J., Pantoja, S., Hughen, K., Lange, C., Gonzalez, F., Munoz, P., Rebolledo, L., Castro, R., Contreras, S., Avila, A., Rossel, P., Lorca, G., Salamanca, M. And Silva, N. 2005. Fluctuations in export productivity over the last century from sediments of a southern Chilean fjord (44°S). Estuarine, Coastal and Shelf Science 65: 587-600.
Serreze, M.C., Holland, M.M. and Stroeve, J. 2007. Perspectives on the Arctic's shrinking sea-ice cover. Science 315: 1533-1536.
Shulenberger, E. and Reid, L. 1981. The Pacific shallow oxygen maximum, deep chlorophyll maximum, and primary production, reconsidered. Deep-Sea Research 28: 901-919.
Smith Jr., W.O. and Comiso, J.C. 2008. Influence of sea ice on primary production in the Southern Ocean: A satellite perspective. Journal of Geophysical Research 113: 10.1029/2007JC004251.
Venrick, E.L., McGowan, J.A., Cayan, D.R. and Hayward, T.L. 1987. Climate and chlorophyll a: Long-term trends in the central North Pacific Ocean. Science 238: 70-72.
Last updated 15 April 2009



